Professional Documents
Culture Documents
Chú Ý Supephotphat Đơn
Uploaded by
Ku NamOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Chú Ý Supephotphat Đơn
Uploaded by
Ku NamCopyright:
Available Formats
-
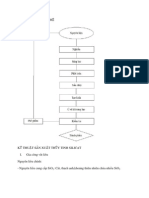
Mono canxi photphat Ca(H2PO4)2 - Canxi sunfat khan CaSO4 - Axit photphoric t do H3PO4 - Photphat st FePO4.2H2O - Photphat nhm AlPO4.2H2O - icanxi photphat CaHPO4 - Apatit cha b phn hy Ca5F(PO4)3 Ngoi ra cn cc mui ca Magie, mt s cht khong trong nguyn liu khng b phn hy, SiO2.nH2O Hin nay supe photphat n sn xut ti Cng ty suphe photphat v ha cht Lm Thao l dng bt ri c trung ha bng chnh qung apatit.
Qung tuyn m: u im: d tch nc gim m c bit l khi c o trn tt. Khi m gim th ti khng dnh bt. Qung tuyn mang i sn xut t tiu chun cht lng: hm lng P2O5 t 32-33%, m <18-22%, kch thc <0.074mm
Tc phn gii giai on II chm v ko di do nhng nguyn nhn sau: Nhng ht qung cha phn gii l cc ht c kch thc ln m axit H3PO4 li l axit ch yu.Lng canxi sunfat kt tinh ra qu nhiu lm cho axit H3PO4 kh tip xc vi ht qung.Mono canxi sunfat tan trong dung dch H3PO4 s dn dn to thnh dung dch bo ha,dn n lm gim hot ca ion H+ trong pha lng,v tng nht ca dung dch.Mono canxisunfat kt tinh to thnh v mn bao bc ht qung lm gim s tip xc pha.
IV. Cc yu t nh hng n qu trnh sn xut 1. Nng v nhit ca axit H2SO4: Tc phn hu ca qung ph thuc vo hot ca axit v mc qu bo ho ca n do sn phm phn ng gy nn. Khi nng cao nng axit sunfuric long v khi gim nng ca axit m c th hot ca chng tng ln do tc v mc apatit phn hu tng ln. Dng axit sunfuric c nng thp th mc phn hu qung cao nhng khng cho php v lng nc a vo theo n qu ln do lm sn phm c m cao, sn phm nho khng ng kt c. Khi nng cao nng axit sunfuric th tc phn hu li b chm li v t n cc tiu ri li tng ln. Khi phn hu apatit bng axit c nng cao hn 63% th pha lng nhanh chng b bo ho bi canxisunphat v th m phn ln cc tinh th CaSO4.1/2H2O v CaSO4 kt tinh dng hnh kim mng, chng to thnh lp v bao ph hu nh ton b b mt ca cc ht apatit, phn ng b km li, supe ng khng tt, pha lng nm li trn b mt cc ht rn v sn phm thu dc c tnh cht ho l xu, khng ti xp m b dnh bt. Nu nng axit thp hn 63% th pha lng b bo ho mc nh hn nn tinh th CaSO4 kt tinh
ln hn to thnh v xp lm axit xm nhp vo b mt kh khn hn do vy phn ng tin hnh nhanh, sn phm thu c kh xp Tuy vy x nghip vn dng axit c nng cao hn gim m ca sn phm. Khi nng cao nng axit ban u m ca sn phm b gim do tng c hm lng P2O5. Tuy nhin khi nng cao nng axit sunfuric qu mc li gy nn s to thnh v canxisunphat mn do bo ho ca n trong dung dch ln lm gim tc phn ng phn hu qung, ngoi ra tc phn ng gim cn do hot ca axit m c nh hn i vi qu trnh sn xut lin tc cho php tng nng axit v axit v apatit vo thng trn cng vi khi phn ng dng bn, nng axit sunfuric gim, nng axit photphoric tng. tan ca CaSO4 trong axit photphoric cao hn trong axit sunfuric nn s qu bo ho v kt tinh ca canxisunphat chm hn, lm cho mng canxisunphat xp. Thng thng nng axit trong sn xut lin tc ln hn gin on 5-7% Nhit ban u ca axit cng nh hng ti vn tc phn hu. Khi nhit tng th tc phn ng ban u tng nhng do qu trnh bo ho canxisunphat nhanh to thnh mng ngn cch, tc phn ng b gim mnh. T thc nghim ta lp c s tng quan gi nng v nhit axit nh sau: Nng axit sunfuric (%khi lng) Nhit (C) 64 65-75 65.5 60-70 67 55-65 68.5 50-60 Tuy nhin nhit axit cng ph thuc vo nhit mi trng. Vi iu kin ca nh my th nng axit thch hp l 66-68% v nhit t 55-75% 2. mn ht qung Qung cng mn th b mt ring cng ln do tc phn hu cng nhanh. Tuy nhin nu qung qu mn th chi ph cho vic sy nghin s tn km nh hng ti gi thnh. 3. Cng khuy trn Vic khuy trn lm mt kh nng bo ho cc b, to s tip xc pha tt hn, do tng cng khuy trn s tng tc phn ng. Nhng khi khuy trn mnh qu th s c st gia qung v axit km i lm gim hiu sut phn ng. Gia cng khuy trn v mn c mi lin h cht ch vi nhau 4. Thi gian lu ca bt st trong thng trn Tu thuc vo thnh phn qung v nng axit a vo phn hu v c khng ch bng tm chn thng trn Do cung cp qung v axit lin tc ng thi bn tan thnnh khng ngng chy qua mt tm chn s gi cho bt st c 1 th tch khng i trong thit b trn, thi gian lu li ca n khng ln trnh cho bn c st lm mt linh ng Nng axit ban u cng cao, mc phn hu qung cng ln th phi duy tr t l H2SO4 : H3PO4 trong bn t thng trn phi cng nh i trnh to v CaSO4. 5. Qu trnh supephotphat kho Supephotphat ct t phng ho thnh ra c cha mt lng apatt cha phn hu. S phn hu tip theo gia apatit v axit photphoric vn tip tc xy ra vi tc d gim dn. Qua nhiu ngy supe trong l h s phn hu tng ln.
h s phn hu tng ta phi h thp nhit xung 40-50C bi khi lm ngui th Ca(H2PO4)2. H2O s kt tinh ra khi pha lng, bo ho pha lng lm gim hot axit v lm tng tc phn ng. Thc t ngi ta dng phng php nh tung supephotphat nhm mc ch: - Lm ngui supe n nhit thch hp - Hi nc v kh Flo d thot ra lm gim m ca supe v h nhit - Supe c trn u, ti, xp v ng nht hn do tnh cht l ho ca supe tng ln 6. Trung ho supephotphat Supe ti ra khi phng ho thnh cn cha 1 lng axit H3PO4 t do ng vi hm lng P2O5 t do c trong supe t 10.5-12.5% khi lng, s tn ti H3PO4 trong supe lm cho sn phm c tnh ht m lm nh hng xu n tnh cht vt l ca sn phm, lm n mn, ph hu phng tin vn chuyn n. Do phi trung ho lng axit t do ny bng bt xng, bt apatit, bt vi, bt lmit, Cc cht c cha photphat khi s dng trung ho s lm tng hm lng P2O5 hu hiu trung ho l tt nht. Mt khc lng apatit cn li b bao bc bi mng CaSO4 v Ca(H2PO4)2. H2O do phn ng gia 2 pha L:R b cn tr. V vy ta s dng qung apatit trung ho supe ti (qung tuyn) Khi trung ho bng cc cht khng cha photpho th hm lng P2O5 hu hiu tng. Nu lng cht trung ho d th hm lng P2O5 gim. Khi trung ho ng liu lng apatit, supephotphat ca cng ty t tiu chun sau: - Hm lng P2O5 hu hiu: 16% - Hm lng P2O5 t do: 4% - m: 13%
You might also like
- Câu Hỏi Liên Quan TN HHCDocument7 pagesCâu Hỏi Liên Quan TN HHCHeo AnNo ratings yet
- KTSX Thuy Tinh SilicatDocument5 pagesKTSX Thuy Tinh SilicatJessica TrevinoNo ratings yet
- Cau Hoi Chuong 3.2. (PP Vôi Và Sulfit)Document4 pagesCau Hoi Chuong 3.2. (PP Vôi Và Sulfit)minh Tom33% (3)
- Phân Bón Cuối KỳDocument12 pagesPhân Bón Cuối KỳHào HếuNo ratings yet
- PHƯƠNG PHÁP ƯỚTDocument2 pagesPHƯƠNG PHÁP ƯỚTLe Ho Chon DuyenNo ratings yet
- The Manufacture of Sulfuric Acid and SupDocument9 pagesThe Manufacture of Sulfuric Acid and Supplinh12205No ratings yet
- Chất Lượng Môi Trường ĐấtDocument3 pagesChất Lượng Môi Trường ĐấtLEGGO XăngNo ratings yet
- Hoá Vô CơDocument20 pagesHoá Vô CơDương MinhNo ratings yet
- Báo cáo thí nghiệm bài 3Document7 pagesBáo cáo thí nghiệm bài 3tit tutNo ratings yet
- DÀN Ý H3PO4 (2)Document20 pagesDÀN Ý H3PO4 (2)Le Ho Chon DuyenNo ratings yet
- Bai 3 JartestDocument15 pagesBai 3 Jartestaveiro261100% (2)
- Ôn Tập Thực Tập Hóa Vô Cơ 1Document13 pagesÔn Tập Thực Tập Hóa Vô Cơ 1Hoàng Dung NguyễnNo ratings yet
- Hoa Huu CoDocument26 pagesHoa Huu CoYenan TruongNo ratings yet
- ĐG BK CK FinalllDocument117 pagesĐG BK CK FinalllUyên MỹNo ratings yet
- đồ án nhập mônDocument4 pagesđồ án nhập mônKiên Đinh HoàngNo ratings yet
- TT Hóa Dư C 2Document3 pagesTT Hóa Dư C 2Nhật Kiều MinhNo ratings yet
- TTHC 2Document7 pagesTTHC 2Võ Thùy DươngNo ratings yet
- Quy trình đường mía và điều kiệnDocument3 pagesQuy trình đường mía và điều kiệnĐinh Lê HoàngAnhNo ratings yet
- Quy Trình Sản Xuất Axit Sunfuric Đi Từ Lưu Huỳnh Nguyên TốDocument46 pagesQuy Trình Sản Xuất Axit Sunfuric Đi Từ Lưu Huỳnh Nguyên TốMinhTrangNguyễn75% (4)
- acid citricDocument26 pagesacid citricppthao1100No ratings yet
- Mạ hóa học NikenDocument4 pagesMạ hóa học NikenLê Huy100% (2)
- Lý thuyết THHC1Document6 pagesLý thuyết THHC1Tôi TellNo ratings yet
- Câu hỏi ôn tập đường bánh kẹo (nmd)Document13 pagesCâu hỏi ôn tập đường bánh kẹo (nmd)nguyenmongduyNo ratings yet
- Jar TestDocument19 pagesJar TestDaisy100% (3)
- Ilovepdf MergedDocument35 pagesIlovepdf MergedThu GiangNo ratings yet
- TT Hóa Dư C 1Document8 pagesTT Hóa Dư C 1Lệ hồNo ratings yet
- Bia Chương XiDocument27 pagesBia Chương XiQuốc TânNo ratings yet
- Bài 4 Định Lượng Đường Khử Trong Hoa Quả Tươi- Môn thực hành phân tích thực phẩmDocument6 pagesBài 4 Định Lượng Đường Khử Trong Hoa Quả Tươi- Môn thực hành phân tích thực phẩmNam NguyenHoangNo ratings yet
- phân tích quy trình công nghệ làm sạch nước mía bằng phương pháp gia vôiDocument29 pagesphân tích quy trình công nghệ làm sạch nước mía bằng phương pháp gia vôiKhanh Nguyen Quoc100% (1)
- BÁO-CÁO-HÓA-SINH-BÀI-3Document8 pagesBÁO-CÁO-HÓA-SINH-BÀI-3nguyenhieuvb04No ratings yet
- QUY TRÌNH SẢN XUẤT ACID ACETIC TỪ ETHANOL BẰNG PHƯƠNG PHÁP LÊN MENDocument6 pagesQUY TRÌNH SẢN XUẤT ACID ACETIC TỪ ETHANOL BẰNG PHƯƠNG PHÁP LÊN MENTran ThuHueNo ratings yet
- Câu hỏi TN Hóa Dược 1Document9 pagesCâu hỏi TN Hóa Dược 1Hạ ĐinhNo ratings yet
- TerpineolDocument3 pagesTerpineolHƯNG LIÊU MẠNHNo ratings yet
- BC thử việc tuần 07 - Nguyễn Bá Khánh TrânDocument17 pagesBC thử việc tuần 07 - Nguyễn Bá Khánh TrânKane100% (1)
- Bai 10. Tong Hop Muoi MohrDocument4 pagesBai 10. Tong Hop Muoi MohrBùi ThươngNo ratings yet
- Lam Sach Nuoc Mia - 2Document41 pagesLam Sach Nuoc Mia - 2Hồ Ái LinhNo ratings yet
- Thi Nghiem Thuc Hanh Vo Co 1Document36 pagesThi Nghiem Thuc Hanh Vo Co 1hoahoc_lang0% (2)
- Bai 10. Tong Hop Muoi Mohr PDFDocument3 pagesBai 10. Tong Hop Muoi Mohr PDFQuỳnh LêNo ratings yet
- Bài 3 TNHSDocument6 pagesBài 3 TNHSMai PhạmNo ratings yet
- NTU - Xử lý RFCC Naphtha trong công nghiệp chế biến dầu khíDocument13 pagesNTU - Xử lý RFCC Naphtha trong công nghiệp chế biến dầu khíVĂN ĐOÀN HUYNo ratings yet
- Bài 3Document5 pagesBài 3Hien DaoNo ratings yet
- 14.Đại cương về ximăng và thành phần, qui trình sản xuất xi măng, xác định độ ẩm và hàm lượng mất khi nung, hàm lượng SiO2 trong ximăngDocument26 pages14.Đại cương về ximăng và thành phần, qui trình sản xuất xi măng, xác định độ ẩm và hàm lượng mất khi nung, hàm lượng SiO2 trong ximăngthjenlong142100% (3)
- - Các biến đổi công đoạn hấpDocument3 pages- Các biến đổi công đoạn hấpAnh QuỳnhNo ratings yet
- Báo cáo thí nghiệm bài đường khử tổng sốDocument4 pagesBáo cáo thí nghiệm bài đường khử tổng sốLê Mai LoanNo ratings yet
- ÔN TẬP HÓA HỌC THỰC PHẨMDocument27 pagesÔN TẬP HÓA HỌC THỰC PHẨMNguyen Thuy Bao Ngoc B2107502No ratings yet
- Nhũ Tương NH A Đư NGDocument4 pagesNhũ Tương NH A Đư NGVăn Bão TôNo ratings yet
- Tài Liệu4 1Document110 pagesTài Liệu4 1hinox9867No ratings yet
- Báo Cáo Thí Nghiệm Hóa Sinh Bài 1: Định Tính Và Khảo Sát Protein Nhóm: S6 - N3 Ngày làm thí nghiệm: 29/04/2022 Thành viên - MSSVDocument6 pagesBáo Cáo Thí Nghiệm Hóa Sinh Bài 1: Định Tính Và Khảo Sát Protein Nhóm: S6 - N3 Ngày làm thí nghiệm: 29/04/2022 Thành viên - MSSVDara LameNo ratings yet
- câu hỏi b5 1-5Document2 pagescâu hỏi b5 1-5Anh MaiNo ratings yet
- Ôn Ly Thuyêt HhcoDocument15 pagesÔn Ly Thuyêt HhcoPham Van Tin B1909842No ratings yet
- SƠ ĐỒ SẢN XUẤT ĐƯỜNG PHÈN CÔNG NGHIỆPDocument6 pagesSƠ ĐỒ SẢN XUẤT ĐƯỜNG PHÈN CÔNG NGHIỆPVi Lê Nguyễn TườngNo ratings yet
- Phan LanDocument2 pagesPhan LanLuong Ha GiangNo ratings yet
- Liên kiềuDocument18 pagesLiên kiềuPhan Như RiNo ratings yet
- Báo Cáo Thí Nghiệm Bài 3 (1)Document8 pagesBáo Cáo Thí Nghiệm Bài 3 (1)Đoàn QuânNo ratings yet
- PHƯƠNG PHÁP ĐIỀU CHẾ CỒN KHÔDocument2 pagesPHƯƠNG PHÁP ĐIỀU CHẾ CỒN KHÔHoa TrịnhNo ratings yet
- Quy Trình SX Rư U VangDocument5 pagesQuy Trình SX Rư U Vangvinh lêNo ratings yet
- PP NhiệtDocument4 pagesPP NhiệtNguyen Thanh VyNo ratings yet
- UD CR Trong CN VliệuDocument1 pageUD CR Trong CN VliệuKu NamNo ratings yet
- (Vnmath - Com) - Bo de Thi Va Kiem Tra Toan 9Document21 pages(Vnmath - Com) - Bo de Thi Va Kiem Tra Toan 9Ku NamNo ratings yet
- Luyen Thi Vao Lop 10 - VVBDocument27 pagesLuyen Thi Vao Lop 10 - VVBNguyen Duy KhanhNo ratings yet
- 3000 TuphienamvagiainghiaDocument95 pages3000 Tuphienamvagiainghiamynga.qt3219No ratings yet
- Xemina Dlcmdcs 8338Document2 pagesXemina Dlcmdcs 8338Ku NamNo ratings yet
- Ky Thuat Thiet Bi Phan UngDocument70 pagesKy Thuat Thiet Bi Phan UngKu NamNo ratings yet
- Chu Truong Cua Dang Trong Giai Quyet Cac Van de Xa HoiDocument6 pagesChu Truong Cua Dang Trong Giai Quyet Cac Van de Xa Hoimet110No ratings yet
- CTDT K53 CNHHDocument96 pagesCTDT K53 CNHHMinh Nguyen VanNo ratings yet
- KHK 5253 NH 2011Document99 pagesKHK 5253 NH 2011Ku NamNo ratings yet