Professional Documents
Culture Documents
MLE1101 Tutorial 4 - Suggested Solutions Analysis
Uploaded by
Yin HauOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
MLE1101 Tutorial 4 - Suggested Solutions Analysis
Uploaded by
Yin HauCopyright:
Available Formats
1
MLE1101 Tutorial 4 - Suggested Solutions
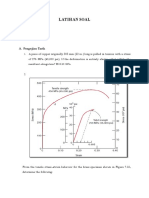
1. The following engineering stress-strain data were obtained at the beginning of a tensile test
for a 0.2%C plain-carbon steel.
(a) Plot the engineering stress-strain curve for these data.
(b) Determine the 0.2% offset yield stress for this steel.
(c) Determine the tensile elastic modulus of this steel. (Note that these data only give
the beginning part of the stress-strain curve.)
(Leave your answers in terms of pounds and inches.)
Engineering
Stress (kpsi)
Engineering
Strain (in./in.)
Engineering Stress
(kpsi)
Engineering
Strain (in./in.)
0 0 60 0.0035
15 0.0005 66 0.004
30 0.001 70 0.006
40 0.0015 72 0.008
50 0.0020
Solution:
(a)
(b) From figure in part (a), 0.2 percent yield stress = 66 kpsi.
(c) From figure in part (a),
7
30 1000 psi
tensile elastic modulus 3.0 10 psi
0.001
E
o
c
= = =
2
2. A 0.505-in.-diameter aluminum alloy test bar is subjected to a load of 25,000 lb
f
. If the
diameter of the bar is 0.490 in. at this load, determine (a) the engineering stress and strain
and (b) the true stress and strain. (Leave your answers in terms of pounds and inches.)
Solution:
( )
( )
2
2
2 0
0
2
2
2
0.505in
0.200in
4 4
0.490in
0.189in
4 4
i
i
d
A
d
A
t t
t t
= = =
= = =
Assume no volume change,
0 0 i i
A l Al = ,
(a)
2
0
25000lb
125,000 psi
0.200in
f
F
A
o = = =
c
= = = =
2
i 0 0
2
0 i
0.200in
1 1 0.058
0.189in
l l A
l A
(b)
2
25000lb
133,000 psi
0.189in
f
T
i
F
A
o = = =
c = = = =
2
0 i
2
0
0.200in
ln ln ln 0.057
0.189in
T
i
A l
l A
3. (a) Why do pure FCC metals like Ag and Cu have low values of critical resolved shear
stress t
c
?
(b) What is believed to be responsible for the high values of t
c
for HCP titanium?
Solution:
(a) For FCC metals like Ag and Cu, slip takes place on the close-packed {111} octahedral
planes and in the 110 < > close packed directions. Lower shear stress is required for
slip to occur in densely packed planes.
(b) The high t
c
values associated with HCP titanium is attributed to the mixed covalent
and metallic bonding with the atomic lattice structure.
3
4. A stress of 75 MPa is applied in the [001] direction on an FCC single crystal. Calculate (a) the
resolved shear stress acting on the (111)[101]
slip system and (b) the resolved shear stress
acting on the (111)[110]
slip system.
Solution:
(a) Referring to figures (i) to (iii) below, = 45, cos 54.7
3
a
a
| | = =
resolved shear stress, cos cos 75cos45 cos54.7 30.6MPa
r
t o | = = =
(b) Referring to figures (i) and (ii) below, = 90, cos 54.7
3
a
a
| | = =
resolved shear stress, cos cos 75cos90 cos54.7 0MPa
r
t o | = = =
4
5. A 70%Cu30%Zn brass wire is cold-drawn 20 percent to a diameter of 2.80 mm. The wire
is then further cold-drawn to a diameter of 2.45 mm. Definition of cold reduction is given as:
change in cross-sectional area
% cold reduction 100%
original cross-sectional area
=
(a) Calculate the total percent cold work that the wire undergoes.
(b) Estimate the wires tensile and yield strengths and elongation from Figure (a) in the
appendix.
Solution:
(a)
change in cross-sectional area
% cold reduction 100%
original cross-sectional area
=
( )
2
2
1 4 4
2
1 4
2 2 2
1 1 1
2.80mm
20% 100%
0.20 7.84mm 3.13mm
d
d
d d d
t t
t
=
= =
( ) ( )
( )
2 2
4 4
2
4
3.13mm 2.45mm
total % cold work 100% 38.75%
3.13mm
t t
t
(
= = (
(
(b) From fig. (a) in appendix, for cold work 39%,
Tensile Strength ~ 76 kpsi; Yield Strength ~ 64 kpsi and Elongation ~ 7 %
6. Derive the lever rule for the amount in weight percent of each phase in two-phase regions of
a binary phase diagram. Use a phase diagram in which two elements are completely soluble
in each other.
Solution:
5
Recognise that the sum of weight fractions of liquid and solid phases is equal to 1
1
l S
X X + =
Considering the weight balance of B in the alloy as a whole and the sum of B in the two
phases, we arrive at:
( )
0
0
0
1
wt fraction of solid phase,
l l S S
S l S S
l
S
S l
w X w X w
w X w X w
w w
X
w w
= +
= +
Similarly,
wt fraction of liquid phase,
S o
l
S l
w w
X
w w
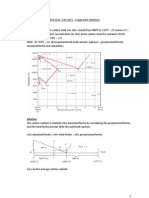
7. Consider the binary eutectic copper-silver phase diagram in the appendix (Figure b). Make
phase analyses of an 88wt%Ag12wt%Cu alloy at the temperatures (a) 1000
o
C, (b) 800
o
C, (c)
780
o
C + AT, (d) 780
o
C AT. AT is assumed to be less than 1
o
C. In the phase analyses, include:
(i) The phases present; (ii) The chemical compositions of the phases; (iii) The amounts of
each phase; (iv) Sketch the microstructure by using 2-cm-diameter circular fields.
Solution:
(a) at 1000
o
C,
Phases present: Liquid
Composition of phase: 88 wt% Ag
Amount of each phase:
wt% of liquid phase = 100%
(b) at 800
o
C,
Phases present: Liquid beta
Composition of phase: 78 wt% Ag in liquid phase 93 wt% Ag in beta phase
Amount of each phase:
93 88
wt% of liquid phase 100% 33.3%
93 78
= =
88 78
wt% of beta phase 100% 66.7%
93 78
= =
6
(c) at 780
o
C + AT,
Phases present: Liquid beta
Composition of phase: 71.9 wt% Ag in liquid phase 91.2 wt% Ag in beta phase
Amount of each phase:
91.2 88
wt% of liquid phase 100% 16.6%
91.2 71.9
= =
88 71.9
wt% of beta phase 100% 83.4%
91.2 71.9
= =
(d) at 780
o
C - AT,
Phases present: alpha beta
Composition of phase: 7.9 wt% Ag in alpha phase 91.2 wt% Ag in beta phase
Amount of each phase:
91.2 88
wt% of alpha phase 100% 3.8%
91.2 7.9
= =
88 7.9
wt% of beta phase 100% 96.2%
91.2 7.9
= =
8. Consider the binary peritectic iridium-osmium phase diagram in the appendix (Figure c).
Make phase analyses of a 70wt%Ir30wt%Os alloy at the temperatures (a) 2600
o
C, (b)
2665
o
C AT, (c) 2665
o
C + AT. AT is assumed to be less than 1
o
C. In the phase analyses,
include: (i) The phases present; (ii) The chemical compositions of the phases; (iii) The
amounts of each phase; (iv) Sketch the microstructure by using 2-cm-diameter circular
fields.
Solution:
(a) at 2600
o
C,
Phases present: Liquid alpha
Composition of phase: 18 wt% Os in liquid phase 40 wt% Os in alpha phase
Amount of each phase:
= =
40 30
wt% of liquid phase 100% 45.4%
40 18
= =
30 18
wt% of alpha phase 100% 54.6%
40 18
7
(b) at 2665
o
C - AT,
Phases present: Liquid alpha
Composition of phase: 23.0 wt% Os in liquid phase 43.0 wt% Os in alpha phase
Amount of each phase:
43 30
wt% of liquid phase 100% 65.0%
43 23
= =
30 23
wt% of alpha phase 100% 35.0%
43 23
= =
(c) at 2665
o
C + AT,
Phases present: Liquid beta
Composition of phase: 23.0 wt% Os in liquid phase 61.5 wt% Os in beta phase
Amount of each phase:
61.5 30
wt% of liquid phase 100% 81.8%
61.5 23
= =
30 23
wt% of beta phase 100% 18.2%
61.5 23
= =
9. Consider an Fe 4.2wt%Ni alloy (figure d in appendix) that is slowly cooled from 1550
o
C to
1450
o
C. Determine the amount of o phase and phase, respectively, at a temperature of
1517
o
C AT. Assume AT to less than 1
o
C.
Solution:
4.2 4
wt% of phase 100% 66.7%
4.3 4
= =
o
= =
4.3 4.2
wt% of phase 100% 33.3%
4.3 4
You might also like
- EN380 Homework #6 Solution: 1084 C 1455 C 70% N i L + α C = 61% C = 73% W = = = 25% W = = = 75%Document4 pagesEN380 Homework #6 Solution: 1084 C 1455 C 70% N i L + α C = 61% C = 73% W = = = 25% W = = = 75%roseNo ratings yet
- Assignment Shaft DesignDocument4 pagesAssignment Shaft DesignHasham Mehmood100% (1)
- HMT Tut1Document2 pagesHMT Tut1Meet ShahNo ratings yet
- CCT Phase Diagram AssignmentDocument4 pagesCCT Phase Diagram AssignmentAyu Sekar TunjungNo ratings yet
- PM P1 D5 - Problemas Deformación PlásticaDocument4 pagesPM P1 D5 - Problemas Deformación PlásticaAnggieGarcésNo ratings yet
- VelocitytutorialDocument4 pagesVelocitytutorialManoz Thapa KajiNo ratings yet
- 27401C Introduction To Heat Treating - ASMDocument283 pages27401C Introduction To Heat Treating - ASMwulfgang66No ratings yet
- Cmi215.2017 - Guia TTDocument9 pagesCmi215.2017 - Guia TTalex123456789009850% (2)
- HW 5Document8 pagesHW 5Anonymous AjrDxGNo ratings yet
- HW 4Document4 pagesHW 4jackalscream91No ratings yet
- SampleqsDocument8 pagesSampleqstommtommNo ratings yet
- 11 Askeland ChapDocument12 pages11 Askeland Chapsergalan100% (1)
- Preguntas Cap 4 Manufactura ModernaDocument13 pagesPreguntas Cap 4 Manufactura ModernaJavierNo ratings yet
- Engineering Materials Phase DiagramsDocument6 pagesEngineering Materials Phase DiagramsOmar AssalNo ratings yet
- GL PMG 1000 Specification SheetDocument1 pageGL PMG 1000 Specification SheetBharat JainNo ratings yet
- Heat Transfer Homework AssignmetDocument4 pagesHeat Transfer Homework Assignmetedison navarroNo ratings yet
- Fulltext 7499Document239 pagesFulltext 7499Jenn QuintoNo ratings yet
- Solidification and Crystalline ImperfectionsDocument43 pagesSolidification and Crystalline ImperfectionsArnaldo Bester67% (3)
- IES Conventional Mechanical Engineering 2017 PAPER 2Document6 pagesIES Conventional Mechanical Engineering 2017 PAPER 2ravi khannaNo ratings yet
- 344W13FinalExam Solution PDFDocument22 pages344W13FinalExam Solution PDFTysir SarhanNo ratings yet
- 15me53 Important Questions Paper-3Document3 pages15me53 Important Questions Paper-3Karthi SiddhNo ratings yet
- Latihan SoalDocument3 pagesLatihan SoalDaus100% (1)
- Atomic and Ionic Arrangements CalculationsDocument19 pagesAtomic and Ionic Arrangements CalculationsRafael AraújoNo ratings yet
- Assignment 4Document10 pagesAssignment 4mahmoud EissaNo ratings yet
- Problems: 7.17 ReferencesDocument2 pagesProblems: 7.17 ReferencesAhmad Shuaib Khawari ,141305No ratings yet
- Worksheet 5 Solution SN Curve Practice ProblemDocument2 pagesWorksheet 5 Solution SN Curve Practice ProblemWolf LordNo ratings yet
- Homework 2 2019Document1 pageHomework 2 2019Kerem Taha Tekin0% (1)
- 14 2042015Assignment14SolutionDocument5 pages14 2042015Assignment14SolutionInfo Esocket100% (1)
- Quiz Show FileDocument18 pagesQuiz Show FileLeaniel SilvaNo ratings yet
- D 4762 - 04 Rdq3njiDocument16 pagesD 4762 - 04 Rdq3njicristina ramosNo ratings yet
- Assignment 2 - 2023 - SolutionsDocument23 pagesAssignment 2 - 2023 - SolutionsLinhan ChuNo ratings yet
- Problems Phase FeDocument9 pagesProblems Phase FeAshutoshKumarNo ratings yet
- 08 Askeland Chap PDFDocument12 pages08 Askeland Chap PDFMaiefnbNo ratings yet
- Calculate Vacancy Formation Energies and Concentrations in SolidsDocument3 pagesCalculate Vacancy Formation Energies and Concentrations in SolidsDavid Sahry Mark100% (1)
- Turning and milling operation time and cost calculationsDocument4 pagesTurning and milling operation time and cost calculationsDominique SmoyerNo ratings yet
- Construction Materials Density and Dimensional ChangesDocument4 pagesConstruction Materials Density and Dimensional ChangesIngrid Zetina Tejero100% (1)
- Problem Set 3Document2 pagesProblem Set 3Dniel Suarez RNo ratings yet
- DEE - 310 - NotesDocument41 pagesDEE - 310 - NotesAlfred K ChilufyaNo ratings yet
- Aerodynamics Assignment On Air-FoilsDocument14 pagesAerodynamics Assignment On Air-Foilsashish choudharyNo ratings yet
- 2162 - Ch3 - Review Questions & Solutions - AY20202021Document20 pages2162 - Ch3 - Review Questions & Solutions - AY20202021lalalalaNo ratings yet
- Defect ProblemsDocument8 pagesDefect Problemsndreddy_pu100% (2)
- HW 07Document8 pagesHW 07Aeyrul KhairulNo ratings yet
- Heat TransferDocument4 pagesHeat TransferRoem EsmernaNo ratings yet
- Furnace Atmospheres No. 1Document36 pagesFurnace Atmospheres No. 1Wilmer Quezada MurilloNo ratings yet
- Saybolt Viscometer CheckedDocument6 pagesSaybolt Viscometer CheckedbjmarsoniaNo ratings yet
- Wilson Conversion ChartDocument1 pageWilson Conversion ChartGuru SamyNo ratings yet
- Orange Peel Grabs PDFDocument13 pagesOrange Peel Grabs PDFweffwdfNo ratings yet
- Phase Diagram Exercises - Worked Answers - CorrectedDocument14 pagesPhase Diagram Exercises - Worked Answers - CorrectedHayden Reeves75% (4)
- Week 11Document12 pagesWeek 11lduran_63No ratings yet
- Calculate phase amounts in Cu-Ni and Pb-Sn alloysDocument8 pagesCalculate phase amounts in Cu-Ni and Pb-Sn alloysBukti NegalNo ratings yet
- Gate 2004 PDFDocument37 pagesGate 2004 PDFAkash Km SinghNo ratings yet
- Che 3330 - Spring 2012 HW 5Document5 pagesChe 3330 - Spring 2012 HW 5Brett CasserlyNo ratings yet
- Chapt 08Document21 pagesChapt 08Jesse McClure100% (5)
- L7 Txy Graph Lecture NotesDocument15 pagesL7 Txy Graph Lecture NotessarahjheehNo ratings yet
- Tutorial 2 HelperDocument3 pagesTutorial 2 HelperDedy SaputraNo ratings yet
- PMMA Stress-Strain AnalysisDocument5 pagesPMMA Stress-Strain AnalysisAnam PirachaNo ratings yet
- Mechanical Engineering Objective Type Questions For ExamsDocument6 pagesMechanical Engineering Objective Type Questions For Examsmanishsingh1067% (3)
- Regression Practice Questions 2024Document3 pagesRegression Practice Questions 2024Brian SamendeNo ratings yet
- Solving Partial Differential Equation Applications with PDE2DFrom EverandSolving Partial Differential Equation Applications with PDE2DNo ratings yet
- Reviews in Computational ChemistryFrom EverandReviews in Computational ChemistryAbby L. ParrillNo ratings yet
- Gek 1540 Tut 2 QBDocument2 pagesGek 1540 Tut 2 QBYin HauNo ratings yet
- Laplace Transform TableDocument3 pagesLaplace Transform TableYin HauNo ratings yet
- Thermodynamics and Its Application in RefrigerationDocument8 pagesThermodynamics and Its Application in RefrigerationYin HauNo ratings yet
- CA Lab Manual ScopeDocument10 pagesCA Lab Manual Scopea2367916100% (1)
- LSM1301Document7 pagesLSM1301Yin HauNo ratings yet
- Entropy 15 01221Document11 pagesEntropy 15 01221Yin HauNo ratings yet
- Engineers & EnvironmentDocument8 pagesEngineers & EnvironmentYin HauNo ratings yet
- Mechanical Properties and Testing of Materials Chapter 2Document7 pagesMechanical Properties and Testing of Materials Chapter 2Yin HauNo ratings yet
- Gek1540 Chapter 6Document4 pagesGek1540 Chapter 6Yin HauNo ratings yet
- ME2142E Feedback Control Systems-CheatsheetDocument2 pagesME2142E Feedback Control Systems-CheatsheetPhyo Wai Aung67% (9)
- Tutorial 7 Suggested AnswersDocument4 pagesTutorial 7 Suggested AnswersYin HauNo ratings yet
- Heat Transfer Radiation Problem SetDocument1 pageHeat Transfer Radiation Problem SetLakshmi BalasubramaniamNo ratings yet
- MNO1001X Cheat SheetDocument8 pagesMNO1001X Cheat SheetYin HauNo ratings yet
- TimetableDocument2 pagesTimetableYin HauNo ratings yet
- Tutorial 7 Suggested AnswersDocument4 pagesTutorial 7 Suggested AnswersYin HauNo ratings yet
- 12 TransientDocument39 pages12 TransientYin HauNo ratings yet
- Heat Transfer Radiation Problem SetDocument1 pageHeat Transfer Radiation Problem SetLakshmi BalasubramaniamNo ratings yet
- ME3112-PART 2 Tutorial 2 & 3: ShahrokhDocument14 pagesME3112-PART 2 Tutorial 2 & 3: ShahrokhYin HauNo ratings yet
- MLE1101 - Tutorial 1 - Suggested SolutionsDocument5 pagesMLE1101 - Tutorial 1 - Suggested SolutionsYin HauNo ratings yet
- Chapter 15Document2 pagesChapter 15Yin HauNo ratings yet
- MLE1101 - Tutorial 6 - Suggested SolutionsDocument5 pagesMLE1101 - Tutorial 6 - Suggested SolutionsYin HauNo ratings yet
- ME3162 Questions PDFDocument2 pagesME3162 Questions PDFYin HauNo ratings yet
- MLE1101 - Tutorial 5 - Suggested SolutionsDocument5 pagesMLE1101 - Tutorial 5 - Suggested SolutionsYin HauNo ratings yet
- LSM1301Document7 pagesLSM1301Yin HauNo ratings yet
- MLE1101 - Tutorial 3 - Suggested SolutionsDocument4 pagesMLE1101 - Tutorial 3 - Suggested SolutionsYin HauNo ratings yet
- L2 - LeadershipDocument2 pagesL2 - LeadershipYin HauNo ratings yet
- MLE1101 Tutorial 2 - Suggested Solutions for BCC Crystal Structure, Lattice Constant and Element IdentificationDocument8 pagesMLE1101 Tutorial 2 - Suggested Solutions for BCC Crystal Structure, Lattice Constant and Element IdentificationYin HauNo ratings yet
- EC1301 Mid-Term Exam Questions (09102009 - Make-Up Exam)Document11 pagesEC1301 Mid-Term Exam Questions (09102009 - Make-Up Exam)Yin Hau100% (1)
- EC1301 - Tutorial 4 (14-18 September 2009) - AnswersDocument9 pagesEC1301 - Tutorial 4 (14-18 September 2009) - AnswersYin HauNo ratings yet
- Einforced: Clay and Concrete MasonryDocument647 pagesEinforced: Clay and Concrete Masonryajsniff100% (6)
- A Look at Hot Gas Defrost: Figure 1 Is A Diagram of An Ammonia Air Unit Designed ForDocument1 pageA Look at Hot Gas Defrost: Figure 1 Is A Diagram of An Ammonia Air Unit Designed ForBen MusimaneNo ratings yet
- Ammonia B PDFDocument9 pagesAmmonia B PDFmehrdad_k_rNo ratings yet
- MM2030 July2012 Quiz2Document1 pageMM2030 July2012 Quiz2Arg SreekarNo ratings yet
- Fluid Mechanics Examples Answers GuideDocument16 pagesFluid Mechanics Examples Answers Guidephhgphuc158No ratings yet
- Chapter 3 - Example 3Document6 pagesChapter 3 - Example 3NIBEDITA DEYNo ratings yet
- Assignment 3 Bearing CapacityDocument1 pageAssignment 3 Bearing CapacitymmNo ratings yet
- Finite Volume MethodDocument35 pagesFinite Volume MethodMohammad Reza100% (1)
- Problemario de Balances de Energía en Estado No EstacionarioDocument2 pagesProblemario de Balances de Energía en Estado No Estacionarioxavier escobar hernandezNo ratings yet
- Exergy Analysis of Organic Rankine CycleDocument9 pagesExergy Analysis of Organic Rankine CycleMukul GoyalNo ratings yet
- KTU BTech ME 2019scheme 2019Scheme-MinorsDocument114 pagesKTU BTech ME 2019scheme 2019Scheme-MinorsLearn Mechanical EngineeringNo ratings yet
- Reinforced Concrete Element: Design of Biaxial Bending and CompressionDocument15 pagesReinforced Concrete Element: Design of Biaxial Bending and CompressionZefira WisnaNo ratings yet
- Hydrostatic Bearing SystemsDocument71 pagesHydrostatic Bearing Systems1mmahoneyNo ratings yet
- Rankie Ka ValuesDocument14 pagesRankie Ka ValuesAnonymous lbchQyNo ratings yet
- Lethabo Thermodynamic ModelDocument36 pagesLethabo Thermodynamic ModelSimba Emmanuel NdunaNo ratings yet
- Entropy 17 06412Document21 pagesEntropy 17 06412Meisy RadhistaNo ratings yet
- SK 19 SK 26 (Old)Document13 pagesSK 19 SK 26 (Old)FranNo ratings yet
- Modeling of The Yield Strength of A Stainless Maraging SteelDocument4 pagesModeling of The Yield Strength of A Stainless Maraging SteelFernando HenriqueNo ratings yet
- Process Design and Simulation PDFDocument5 pagesProcess Design and Simulation PDFChemical EngineeringNo ratings yet
- DraftDocument27 pagesDraftisaudkNo ratings yet
- Reservoir EngineeringDocument147 pagesReservoir Engineeringjohn ngandouNo ratings yet
- Out PDFDocument243 pagesOut PDFDoan PhiNo ratings yet
- Mini Project Particle TechDocument10 pagesMini Project Particle TecherickhadinataNo ratings yet
- PHY430 - 433 - Test - 6 February 2021Document7 pagesPHY430 - 433 - Test - 6 February 2021Aidil IkhwanNo ratings yet
- H1 001Document184 pagesH1 001lenova lenovaNo ratings yet
- Raw Mill Heat Balance Without WHR: Sn. Parameters Units ValuesDocument3 pagesRaw Mill Heat Balance Without WHR: Sn. Parameters Units ValuesRamachandrarao MaddaliNo ratings yet
- 4 3qtr Inch PBL ProcedureDocument7 pages4 3qtr Inch PBL ProcedureAnonymous D9jw6iPYEhNo ratings yet
- KR SCAC (R410A 50,60Hz) HP Inverter Brazil MFL69982107 0CSL0-01A (July.2017) PDFDocument91 pagesKR SCAC (R410A 50,60Hz) HP Inverter Brazil MFL69982107 0CSL0-01A (July.2017) PDFFelipe BabugiaNo ratings yet