Professional Documents
Culture Documents
Ap Chemistry Curriculum Map
Uploaded by
api-249441006Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Ap Chemistry Curriculum Map
Uploaded by
api-249441006Copyright:
Available Formats
Curriculum Map: Advanced Placement Chemistry Enduring Understanding/ Essential Questions Numbers refer to Enduring Understandings from the
College Board Course Description NYS Common Core !earning Standards Addressed Numbers refer to Learning Objectives from the College Board Course Description
2ig .dea ,: $he chemical elements are 0undamental #uilding materials o0 matter- and all matter can #e understood in terms o0 arrangements o0 atoms/ $hese atoms retain their identity in chemical reactions/ ,/, 4 ,/5 ,/,6 ,/,7 ,/,8 ,/,9 2ig .dea 3: Chemical and physical properties Use :imensional Analysis to convert units o0 measurement and solve multistep ;uantitative pro#lems/ Separate mi<tures #ased on chemical and physical properties o0 su#stances/ Apply understanding o0 uncertainty in measurement to appropriate use o0 signi0icant 0igures in the la# measurement and calculations/ Assess the value o0 historical e<periments that support our current model o0 the atom/ Inquiry Lab: Food Dyes in Beverages or Separation of Dyes Using Chromatography Per0orm serial dilutions Use spectroscopy to determine the concentration o0 0ood dye%s) in sports drin"s Analy=e spectroscopic measurements and e<trapolate 0rom graphical data >+ .nvestigate the 0actors that in0luence the separation o0 0ood dyes using paper chromatography :esign an e<periment to identi0y a solvent that 'ill give ma<imum resolution o0 a mi<ture o0 dyes :erive a connection #et'een the structure and mo#ililty o0 0ood dyes !a# Port0olio E<it Passes !a# Qui= ? Spectroscopy/Chro matography !a# Qui= ? :etermination o0 Chemical *ormula Pro#lem Set , Pro#lem Set 3 &ome'or" Qui==es %'ee"ly) Unit $ %!am Summative& 2ro'n- !eMay Chapter , Chapter 3 *linn AP Advanced .n;uiry !a#s: ,- 5 College 2oard AP Chemistry @uided4.n;uiry E<periments: ,- 5
S"ills to #e $argeted
Strategies/Activities %&o' 'ill students demonstrate their understanding()
*ormative and Summative Assessments
+esources
Unit ,: Atoms- Molecules- and .ons E/U/ ,/A: All matter is made o0 atoms/ $here are a limited num#er o0 types o0 atoms1 these are the elements/ ,/2: All matter is made o0 atoms/ $here are a limited num#er o0 types o0 atoms1 these are the elements/ ,/E Atoms are conserved in physical and chemical processes/ 3/A Matter can #e descri#ed #y its physical properties/ $he physical properties o0 a su#stance generally depend on the spacing #et'een the particles %atomsmolecules- ions) that ma"e up the su#stance and the 0orces o0 attraction among them/
Lab: Determination of a Chemical Formula Cu!Cly " #
Curriculum Map: Advanced Placement Chemistry
3/2 *orces o0 attraction #et'een particles %including the no#le gases and also di00erent parts o0 some large molecules) are important in determining many macroscopic properties o0 a su#stance- including ho' the o#serva#le physical state changes 'ith temperature/ 3/C $he strong electrostatic 0orces o0 attraction holding atoms together in a unit are called chemical #onds/ 3/: $he type o0 #onding in the solid state can #e deduced 0rom the properties o0 the solid state/ 5/: Electrostatic 0orces e<ist #et'een molecules as 'ell as #et'een atoms or ions- and #rea"ing the resultant intermolecular interactions re;uires energy/ o0 materials can #e e<plained #y the structure and the arrangement o0 atoms- ions- or molecules and the 0orces #et'een them/ 3/, 3/8 3/,A 3/,7 3/,5 3/,8 3/,B '()& Use ;uantitative mass data to determine molar ratios 'ithin a chemical 0ormula
Evaluate the current atomic model Custi0y the arrangement o0 the Periodic $a#le 'ith respect to atomic structure- chemical and physical properties Use the .UPAC system to name chemical compounds/ Compare and contrast the structure and properties o0 molecular and ionic compounds/ Apply atomic molecular theory to Dusti0y the !a' o0 Constant Composition Eisually represent atoms and molecules to demonstrate the !a' o0 Conservation o0 Mass and !a' o0 Constant Composition 2alance chemical e;uations
Unit 3: Stoichiometry Calculations 'ith Chemical
2ig .dea ,: $he chemical elements
Inquiry Lab: *reen Chemistry
!a# Port0olio
2ro'n- !eMay Chapter 6
Curriculum Map: Advanced Placement Chemistry
*ormulas and E;uations ,/A: All matter is made o0 atoms/ $here are a limited num#er o0 types o0 atoms1 these are the elements/ ,/E Atoms are conserved in physical and chemical processes/ 6/A Chemical changes are represented #y a #alanced chemical e;uation that identi0ies the ratios 'ith 'hich reactants react and products 0orm/ 6/2 Chemical reactions can #e classi0ied #y considering 'hat the reactants are- 'hat the products areor ho' they change 0rom one into the other/ Classes o0 chemical reactions include synthesisdecomposition- acid4#ase- and o<idation4reduction reactions/ 6/C Chemical and physical trans0ormations may #e o#served in several 'ays and typically involve a change in energy/ are 0undamental #uilding materials o0 matter- and all matter can #e understood in terms o0 arrangements o0 atoms/ $hese atoms retain their identity in chemical reactions/ ,/,7 ,/,84,/,B 2ig .dea 6: +nalysis of a ,i!ture Use stoichiometry to relate chemical ;uantities in the la# Apply the !a' o0 Conservation o0 Mass and the !a' o0 :e0inite Proportions to analy=e la# data Analy=e ;uantities in the la# related to the mole relationships o0 a #alanced e;uation Convert mass to mole1 mass to particle Calcualate empirical and molecular 0ormulae 0rom mass data Solve limiting reagent pro#lems Calculate theoretical yield #ased on stoichiometry and compare to e<perimental yield :esign and carry out a green chemistry e<periment that can ;uantitatively measure the 'eight percent o0 one compound in a mi<ture o0 t'o compounds Compare atom economy 0rom e<perimental data to theoretical values Assess the procedure design in terms o0 three green principles E<it Passes !a# Qui= ? @reenChemistry Analysis o0 a Mi<ture !a# Qui= ? Stoichiometric :eterminations !a# Qui= ? G Copper in 2rass Pro#lem Set 6 &ome'or" Qui==es %'ee"ly) Unit ( %!am Summative&
&all !a# 2oo": E<periment ,, *linn AP Advanced .n;uiry !a#s: 3- 8 College 2oard AP Chemistry @uided4.n;uiry E<periments: 3- 8
Changes in matter involve the rearrangement and/or reorgani=ation o0 atoms and/or the trans0er o0 electrons/
6/, 6/3 6/6 6/7 6/5 6/F 2ig .dea 5: $he la's o0
Lab: Stoichiometric Determinations %&all !a# 2oo") Use graphical analysis to determine the ;uantities o0 t'o reactants to ma<imum actual product yield :etermine percent yield 0or a reaction #et'een sodium car#onate and hydrochloric acid %#ased on calculated theoretical yield) 2urn magnesium in air and determine the 0ormula 0or magnesium o<ide- #ased on a "no'n ;uantity o0 magnesium and the mass o0 product
Inquiry Lab: - Copper in Brass :esign a procedure to analy=e the amount o0 copper in #rass using visi#le spectroscopy
Curriculum Map: Advanced Placement Chemistry
thermodynamics descri#e the essential role o0 energy and e<plain and predict the direction o0 changes in matter/ 5/,A Construct a cali#ration curve and investigate the concentration range over 'hich 2eerHs la' is valid .denti0y the optimal 'avelength 0or analysis
Unit 6: A;ueous +eactions and Solution Stoichiometry ,/E Atoms are conserved in physical and chemical processes/ 3/A Matter can #e descri#ed #y its physical properties/ $he physical properties o0 a su#stance generally depend on the spacing #et'een the particles %atomsmolecules- ions) that ma"e up the su#stance and the 0orces o0 attraction among them/ 6/A Chemical changes are represented #y a #alanced chemical e;uation that identi0ies the ratios 'ith 'hich reactants react and products 0orm/
2ig .dea ,: $he chemical elements are 0undamental #uilding materials o0 matter- and all matter can #e understood in terms o0 arrangements o0 atoms/ $hese atoms retain their identity in chemical reactions/ ,/,94 ,/3A 2ig .dea 3: Chemical and physical properties o0 materials can #e
Predict the products o0 a chemical reaction given the names o0 the reactants Use visual representations to di00erentiate the #ehavior o0 molecular solids 0rom ionic solids in dissolution Evaluate the electrolytic nature o0 solutions #ased on their chemical composition Predict the products o0 precipitation reactions #ased on solu#ility rules +epresent chemical reactions sym#olically %overall- complete
Lab: *ravimetric +nalysis of Calcium and 'ard .ater/ Analy=e 'ater samples 0or the presence o0 CaC>6 .solate- dry- and 'eigh a precipitate to determine hardness o0 'ater %;uantitative) Use #alanced chemical e;uations to predict the amount o0 precipitate that 'ill #e 0ormed
!a# Port0olio E<it Passes !a# Qui= ? @ravimetric Analysis o0 Calcium and &ard Iater !a# Qui= ? $itration . +eaction Prediction Assignment %:emos) Pro#lem Set 7 &ome'or" Qui==es %'ee"ly) Unit 4 %!am Summative&
2ro'n- !eMay Chapter 7 *linn AP Advanced .n;uiry !a#s: 6 College 2oard AP Chemistry @uided4.n;uiry E<periments: 6
Lab: Standardi#ation of 0a)' Solution1 Strong +cid2Strong Base 3itration Standardi=e a #ase solution 0or use in a strong acid4strong #ase titration Use the endpoint 0rom a strong acid4strong #ase
Curriculum Map: Advanced Placement Chemistry
6/2 Chemical reactions can #e classi0ied #y considering 'hat the reactants are- 'hat the products are- or ho' they change 0rom one into the other/ Classes o0 chemical reactions include synthesisdecomposition- acid4#ase- and o<idation4reduction reactions/ e<plained #y the structure and the arrangement o0 atoms- ionsor molecules and the 0orces #et'een them/ 3/,7 3/,5 2ig .dea 6: Changes in matter involve the rearrangement and/or reorgani=ation o0 atoms and/or the trans0er o0 electrons/ 6/, 4 6/7 6/9 2ig .dea F: Any #ond or intermolecular attraction that can #e 0ormed can #e #ro"en/ $hese t'o processes are in a dynamic competitionsensitive to initial conditions and e<ternal ionic and net ionc) titration to determine the concentration o0 an un"no'n acid +eaction :EM>S ? Students o#serve demonstrations o0 various reaction types and then 'rite appropriately #alanced chemical e;uations/
Standardi=e a #ase Per0orm a strong acid4 strong #ase titration and analy=e the data collected to determine the concentration o0 an un"no'n acid Predict the products o0 acid4#ase neutrali=ation Predict the products o0 o<idation4reduction reactions #ased on a given activity series %Chem & ? students developed the activity series through a la# activity) Assign o<idation num#ers
Apply the concept o0 molarity to solution stoichiometry
Curriculum Map: Advanced Placement Chemistry
pertur#ations/ F/6 F/,, Unit 7: $hermochemistry . 5/A $'o systems 'ith di00erent temperatures that are in thermal contact 'ill e<change energy/ $he ;uantity o0 thermal energy trans0erred 0rom one system to another is called heat/ 5/2 Energy is neither created nor destroyed- #ut only trans0ormed 0rom one 0orm to another/ 5/C 2rea"ing #onds re;uires energyand ma"ing #onds releases energy/ 5/: Electrostatic 0orces e<ist #et'een molecules as 'ell as #et'een atoms or ions- and #rea"ing the resultant intermolecular interactions re;uires energy/ 5/E Chemical or physical processes are driven #y a decrease in enthalpy or an increase in F/,5 2ig .dea 6: Changes in matter involve the rearrangement and/or reorgani=ation o0 atoms and/or the trans0er o0 electrons/ 6/,, 2ig .dea 5: $he la's o0 thermodynamics descri#e the essential role o0 energy and e<plain and predict the direction o0 changes in matter 5/345/9 5/,345/,5 2ig .dea F: Any #ond or intermolecular Apply potential and "inetic energy to chemical systems to descri#e the interactions #et'een atoms- molecules- and su#atomic particles/ +elate the energy o0 particulate interactions to chemical properties Collect and analy=e calorimetry data 'ith respect to system and surroundings %endothermic vs/ e<othermic) Connect energy and enthalpy o0 chemical systems to electrical 'or" and mechanical 'or" done #y e<panding gases Calculate enthalpy o0 reaction 0rom e<perimental data and 0rom Standard Enthalpies o0 *ormation %&essHs !a') *uided Inquiry +ctivity: 3hermochemistry Unit Introduction Students 'ill descri#e the energy e<changes in hot and cold pac"s Inquiry Lab: 'eat of 0eutrali#ation :esign an e<periment to ;uantitatively determine the heat e<changed in a neutrali=ation reaction !a# Port0olio E<it Passes !a# Qui= ? &eat o0 Neutrali=ation !a# Qui= ? :esign o0 a &and'armer Pro#lem Set 5 &ome'or" Qui==es %'ee"ly) Unit 5 %!am Summative& 2ro'n- !eMay Chapter 5 Chapter ,B *linn AP Advanced .n;uiry !a#s: ,3 College 2oard AP Chemistry @uided4.n;uiry E<periments: ,3
Inquiry Lab: Design of a 'and .armer :esign an e00ective hand 'armer that is ine<pensive- nonto<icand sa0e 0or the environment :etermine the heat o0 solution 0or a solid Analy=e cost and sa0ety in0ormation o0 chemical ingredients
Curriculum Map: Advanced Placement Chemistry
entropy- or #oth/ F/A Chemical e;uili#rium is a dynamic- reversi#le state in 'hich rates o0 opposing processes are e;ual attraction that can #e 0ormed can #e #ro"en/ $hese t'o processes are in a dynamic competitionsensitive to initial conditions and e<ternal pertur#ations/ F/,
Analy=e a system to determine 'hether a process is spontaneous or non4 spontaneous :esign conditions that 0avor the 0ormation o0 a products 0or a given system :escri#e the entropy change 0or a given process at the particulate level %use a visual representation) Calculate the *ree Energy Change %@i##s *ree Energy- @) and evaluate the e00ect o0 temperature on @ Use calorimetry data to design a consumer product
Unit 5: Electronic Structure o0 Atoms/Periodic Properties o0 Elements ,/2 $he atoms o0 each element have uni;ue structures arising 0rom interactions #et'een electrons and nuclei/ ,/C Elements display periodicity in their properties
2ig .dea ,: $he chemical elements are 0undamental #uilding materials o0 matter- and all matter can #e understood in terms o0 arrangements o0 atoms/ $hese
Evaluate the current model o0 the atom and contrast 'ith the 2ohr %shell) model o0 the atom Use spectral data to analy=e the energy associated 'ith changing electron position
Lab: 'ydrogen Spectrum Analy=e the &ydrogen spectrum- calculate the amount o0 energy an electron has in a speci0ic or#it and compute the 'avelength o0 the light energy emitted as an electron moves 0rom a higher or#it to a lo'er or#it %#ased
!a# Port0olio E<it Passes !a# Qui= ? &ydrogen Spectrum !a# Qui= ? :esign o0 a &and'armer Pro#lem Set F
2ro'n- !eMay Chapter F Chapter 8 &all !a# 2oo": E<periment ,9
Curriculum Map: Advanced Placement Chemistry
'hen the elements are organi=ed according to increasing atomic num#er/ $his periodicity can #e e<plained #y the regular variations that occur in the electronic structures o0 atoms/ Periodicity is a use0ul principle 0or understanding properties and predicting trends in properties/ .ts modern4day uses range 0rom e<amining the composition o0 materials to generating ideas 0or designing ne' materials/ ,/: Atoms are so small that they are di00icult to study directly1 atomic models are constructed to e<plain e<perimental data on collections o0 atoms/ atoms retain their identity in chemical reactions/ ,/54,/,6 ,/,5 :e0ine an or#ital and develop a relationship #et'een the placement o0 outer electrons and the placement o0 an element on the Periodic $a#le Use electron con0iguration notation to descri#e electrons in a given element Predict the properties o0 elements #ased on position in the Periodic $a#le %atomic radiusioni=ation energyelectron a00inity) E<plain the trends in properties #ased on atomic structure %e00ective nuclear charge- shielding e00ect- etc/) Use !e'is sym#ols as a visual representation o0 #onding %ioniccovalent- metallic)/ Apply the Uncertainty Principle 'ith respect to the 'ave nature o0 the electron to understand the Quantum Mechanical Model o0 the atom on the 2ohr model o0 the atom) &ome'or" Qui==es %'ee"ly) Unit 6 %!am Summative&
Flame 3est Demonstration @iven data 0or ioni=ation energy- melting point- atomic si=e- etc/ @raph the data and e<plain the reason%s) 0or the trend/
Unit F: Chemical 2onding/Molecular @eometry ,/A All matter is made o0 atoms/ $here are a limited
2ig .dea ,: $he chemical elements are 0undamental #uilding
Inquiry Lab: .hat7s in that Bottle8 :esign a procedure to identi0y t'elve un"no'n
!a# Port0olio E<it Passes !a# Qui= ?
2ro'n- !eMay Chapter 9 Chapter B *linn AP
Curriculum Map: Advanced Placement Chemistry
num#er o0 types o0 atoms1 these are the elements/ 3/C $he strong electrostatic 0orces o0 attraction holding atoms together in a unit are called chemical #onds/ 3/: $he type o0 #onding in the solid state can #e deduced 0rom the properties o0 the solid state/ 5/C 2rea"ing #onds re;uires energy- and ma"ing #onds releases energy/ 5/: Electrostatic 0orces e<ist #et'een molecules as 'ell as #et'een atoms or ions- and #rea"ing the resultant intermolecular interactions re;uires energy/ materials o0 matter- and all matter can #e understood in terms o0 arrangements o0 atoms/ $hese atoms retain their identity in chemical reactions/ ,/8 ,/9 2ig .dea 3: Chemical and physical properties o0 materials can #e e<plained #y the structure and the arrangement o0 atoms- ionsor molecules and the 0orces #et'een them/ 3/, 3/,6 3/,8 43/37 3/3F Analy=e the energetics o0 ionic #ond 0ormation %2orn4&a#er Cycle) Use the >ctet +ule and calculation o0 *ormal charge to determine #est !e'is structure representation Use #ond enthalpy and Coulom#Hs !a' to analy=e the strength o0 a covalent #ond Apply Periodicity %electronegativity) to evaluate pond polarity Apply the Ealence Shell Electron Pair +epulsion $heory to determine molecular geometry Evaluate molecular polarity #ased on molecular geometry solids #ased on systematic testing o0 their physical and chemical properties %;ualitative analysis) Select measura#le properties that 'ill help identi0y the type o0 #onding in solids +evie' the properties o0 solids 'ith 0our types o0 chemical #onds %ionicpolar covalent- nonpolar covalent- metallic) #y testing the physical/chemical properties o0 "no'n su#stances IhatHs in that 2ottle( !a# Qui= ? Molecular Models Pro#lem Set 8 &ome'or" Qui==es %'ee"ly) Unit 9 %!am Summative& Advanced .n;uiry !a#s: F College 2oard AP Chemistry @uided4.n;uiry E<periments: F &all !a# 2oo": E<periment ,B
Lab: ,olecular ,odels +ctivity Use molecular model "its to generate a physical representation o0 chemical compounds at the atomic level
Curriculum Map: Advanced Placement Chemistry
3/38 3/3B 2ig .dea 5: $he la's o0 thermodynamics descri#e the essential role o0 energy and e<plain and predict the direction o0 changes in matter 5/, Unit 8: 2ehavior o0 @ases 3/A Matter can #e descri#ed #y its physical properties/ $he physical properties o0 a su#stance generally depend on the spacing #et'een the particles %atomsmolecules- ions) that ma"e up the su#stance and the 0orces o0 attraction among them/ 5/A $'o systems 'ith di00erent temperatures that are in thermal contact 'ill e<change energy/ 5/9 2ig .dea 3: Chemical and physical properties o0 materials can #e e<plained #y the structure and the arrangement o0 atoms- ionsor molecules and the 0orces #et'een them/ 3/, 3/74 3/F Compare and contrast the properties o0 solids- li;uids- and gases :escri#e the deal #ehavior o0 atoms/molecules o0 a gas #ased on JM$ and investigate the properties that results 0rom this atomic #ehavior Use the @as !a's to ;uantitatively analy=e the #ehavior o0 gases under any set o0 conditions %pressureLab: ,olar ,ass of a :olatile Liquid :etermine the molar mass o0 a volatile li;uid #y measuring the mass o0 vapor needed to 0ill a 0las" o0 "no'n volume at a particular temperature and pressure %.deal @as !a'1 PE K n+$) !a# Port0olio E<it Passes !a# Qui= ? Molar Eolume o0 a @as !a# Qui= ? Preparation and Properties o0 Common @ases Pro#lem Set 9 &ome'or" Qui==es %'ee"ly) Unit < %!am &all !a# 2oo" E<periment ,6 E<periment ,7 E<periment ,5 2ro'n- !eMay Chapter ,A
Lab: ;reparation and ;roperties of Common *ases Prepare hydrogen gas-
Curriculum Map: Advanced Placement Chemistry
$he ;uantity o0 thermal energy trans0erred 0rom one system to another is called heat/ 5/2 Energy is neither created nor destroyed- #ut only trans0ormed 0rom one 0orm to another/ 5/: Electrostatic 0orces e<ist #et'een molecules as 'ell as #et'een atoms or ions- and #rea"ing the resultant intermolecular interactions re;uires energy/ 3/,3 2ig .dea 5: $he la's o0 thermodynamics descri#e the essential role o0 energy and e<plain and predict the direction o0 changes in matter 5/3 5/F volume- temperature;uantity) :erive the .deal @as E;uation and derive the 0actors that result in +eal %vs/ .deal) #ehavior in gases Employ the .deal @as E;uation to analy=e la# data 0or determination o0 density and molar mass o0 an un"no'n gas Use Stoichiometry to analy=e Partial Pressure in a mi<ture o0 gases :erive @rahamHs !a' %;ualitatively and ;uantitatively) #ased on data collected in the la# Compare and contrast solids- li;uids- and gases at the particulate level :erive the role o0 temperature and intermolecular 0orces in determining the physical state o0 a su#stance Evaluate the 0our o<ygen gas- and car#on dio<ide gas %products o0 6 di00erent chemical reactions) Collect these gases #y displacement over 'ater .nvestigate the properties o0 each gas to con0irm the reaction products Summative&
*uided Inquiry +ctivity: *raham7s La= of Diffusion :etermine the relative rates o0 di00usion o0 the gases hydrogen chloride and ammonia#y measuring the distances traveled #y the t'o gases in the same time period :erive the @rahamHs !a' relationship #ased on this e<perimental data !a#: Molar Mass #y *ree=ing Point :epression @uided .n;uiry Activity: .ntermolecular AttractionsPolarity- .on :ipole !a# Port0olio E<it Passes !a# Qui= ? Molar Mass #y *ree=ing Point :epression Pro#lem Set B &ome'or" Qui==es 2ro'n- !eMay Chapter ,, Chapter 12 Chapter ,6 &all !a# 2oo" E<periment 36
Unit 9: .ntermolecular *orces %!i;uids- Solids- and Solutions) 3/A Matter can #e descri#ed #y its physical properties/ $he physical properties o0 a su#stance generally depend on the spacing #et'een the particles %atomsmolecules- ions) that ma"e up the su#stance and the 0orces o0
2ig .dea 3: Chemical and physical properties o0 materials can #e e<plained #y the structure and the arrangement o0 atoms- ionsor molecules and the 0orces
Curriculum Map: Advanced Placement Chemistry
attraction among them/ 3/2 *orces o0 attraction #et'een particles %including the no#le gases and also di00erent parts o0 some large molecules) are important in determining many macroscopic properties o0 a su#stance- including ho' the o#serva#le physical state changes 'ith temperature/ 3/C $he strong electrostatic 0orces o0 attraction holding atoms together in a unit are called chemical #onds/ 3/: $he type o0 #onding in the solid state can #e deduced 0rom the properties o0 the solid state/ 5/: Electrostatic *orces e<ist #et'een molecules as 'ell as #et'een atoms or ions- and #rea"ing the resultant intermolecular interactions re;uires energy/ #et'een them/ 3/, 3/6 3/843/,, 3/,F 3/35 3/394 3/63 2ig .dea 5: $he la's o0 thermodynamics descri#e the essential role o0 energy and e<plain and predict the direction o0 changes in matter 5/, 5/B 4 5/,, intermolecular 0orces %dispersion- dipole4 dipole- hydrogen #onds- ion4dipole) 0or a particular su#stance #ased on molecular structure Analy=e the energy changes associated 'ith the phase changes E<amine and descri#e the dynamic e;uili#rium #et'een a li;uid and gaseous state E<plain the variation in vapor pressure 0or given su#stances at the particulate level #ased on molecular structure and intermolecular 0orces Connect the properties o0 metals to structural attri#utes and use representations to e<plain these connections :escri#e the use o0 alloying to modi0y the properties o0 pure metallic elements Use the electron sea %'ee"ly) Unit > %!am Summative&
Curriculum Map: Advanced Placement Chemistry
model to predict or ma"e claims aout the macroscopic properties o0 metals/alloys E<plain the properties o0 molecular solids 'ith respect to the relatively 'ea" intermolecular 0orces Use structural attri#utes to contrast covalent4net'or" solids 'ith other molecular solids Use molecular/ionic structure to descri#e the 0ormation o0 solutions at the particulate level E<plain the 0ormation o0 solutions 'ith respect to the tendency to'ard minimum enthalpy Use structural attri#utes and interactions to predict the 0actors a00ecting solu#ility Use stoichiometry and solution concentration to analy=e solutions ;uantitatively
Curriculum Map: Advanced Placement Chemistry
Use colligative properties to calculate molar mass o0 an un"no'n su#stance Create and interpret representations that lin" the concept o0 molarity 'ith particle vie's o0 solutions Unit B: Chemical Jinetics ,/E Atoms are conserved in physical and chemical processes/ 7/A +eaction rates that depend on temperature and other environmental 0actors are determined #y measuring changes in concentrations o0 reactants or products over time/ 7/2 Elementary reactions are mediated #y collisions #et'een molecules/ >nly collisions having su00icient energy and proper relative orientation o0 reactants lead to products/ 7/C Many reactions proceed via a series o0 elementary reactions/ 7/: +eaction rates may #e increased #y the presence o0 a catalyst/ 2ig .dea ,: $he chemical elements are 0undamental #uilding materials o0 matter- and all matter can #e understood in terms o0 arrangements o0 atoms/ $hese atoms retain their identity in chemical reactions/ ,/,5 ,/,F 2ig .dea 7: +ates o0 chemical reactions are determined #y details o0 the molecular Use representations and the energy pro0ile to predict the temperature dependence 0or a particular reaction Evaluate the validity o0 proposed reaction Analy=e la# data to determine the 0actors that a00ect the rate o0 a chemical reaction %Chemistry &onors !a#) E<plain the 0actors that a00ect the rate o0 a chemical reaction at the particulate level %'ith respect to e00ective collisions) :escri#e e00ective collisions 'ith respect to orientation and energy Inquiry Lab: ?inetics and @ate of @eaction: @ate of decomposition of Calcium Carbonate Collect and measure the volume o0 gas generated #y a heterogeneous reaction o0 calcium car#onate 'ith hydrochloric acid :esign a "inetics e<periment to determine the rate la' 0or a given reaction %graphical analysis) Colla#orate 'ith peers to collect and compare data 0or mass loss and volume o0 gas generated versus time !a# Port0olio E<it Passes !a# Qui= ? Jinetics and +ate o0 +eaction !a# Qui= ? A Jinetic Study %Crystal Eiolet) Pro#lem Set ,A &ome'or" Qui==es %'ee"ly) Unit A %!am Summative& *linn AP Advanced .n;uiry !a#s: ,A- ,, College 2oard AP Chemistry @uided4.n;uiry E<periments: ,A- ,, 2ro'n- !eMay Chapter ,7
Lab: + ?inetic Study: @eaction of Crystal :iolet =ith 0a)' Use spectroscopy and
Curriculum Map: Advanced Placement Chemistry
collisions 7/,47/B Compare and contrast reactions mechanisms and energy pro0ile representations 0or cataly=ed and non4 cataly=ed reactions Analy=e initial rate data %;uantitatively) to determine the order o0 a given reaction E<plain 'hy radioactive decay is classi0ied as a 0irst order reaction Use spectroscopy to analy=e the progress o0 a chemical reaction over time :escri#e the state o0 e;uili#rium 'ith respect to the rate o0 the 0or'ard and reverse reactions Use minimum enthalpy and ma<imum entropy as guiding principles to determine the 0avora#ility o0 a given reaction :esign conditions under 'hich thermodynamically mechanisms 'ith respect to rate data graphical analysis to determine the rate la' 0or the color40ading reaction o0 crystal violet 'ith sodium hydro<ide Construct a cali#ration curve o0 a#sor#ance versus concentration 0or the dye crystal violet @enerate a 2eerHs la' plot to calculate the concentration o0 any Lun"no'nM solution
Light SticB ?inetics
Unit ,A: Chemical E;uili#rium ,/E Atoms are conserved in physical and chemical processes/ 6/A Chemical changes are represented #y a #alanced chemical e;uation that identi0ies the ratios 'ith 'hich reactants react and products 0orm/ F/A Chemical e;uili#rium is a dynamic- reversi#le state in 'hich rates o0 opposing
2ig .dea ,: $he chemical elements are 0undamental #uilding materials o0 matter- and all matter can #e understood in terms o0 arrangements o0 atoms/ $hese atoms retain their identity in chemical reactions/
Lab: Spectrophotometric Determination of an %quilibrium Constant FeSC0C( &
!a# Port0olio E<it Passes !a# Qui= ? Spectrophotome tric :etermination o0 an E;uili#rium !a# Qui= ? Applications o0 !eChatelier Priniciples Pro#lem Set ,,
2ro'n- !eMay Chapter ,5 *linn AP Advanced .n;uiry !a#s: ,6 College 2oard AP Chemistry @uided4.n;uiry E<periments: ,6
*uided Inquiry +ctivity: Le Chatelier7s ;rinciple @iven the stress put on a system at e;uili#riumstudents predict the shi0t and resultant o#servations/
Curriculum Map: Advanced Placement Chemistry
processes are e;ual/ ,/,F F/2 Systems at e;uili#rium are responsive to e<ternal pertur#ations- 'ith the response leading to a change in the composition o0 the system/ 2ig .dea 5: $he la's o0 thermodynamics descri#e the essential role o0 energy and e<plain and predict the direction o0 changes in matter 5/,F 4 5/,9 2ig .dea F: Any #ond or intermolecular attraction that can #e 0ormed can #e #ro"en/ $hese t'o processes are in a dynamic competitionsensitive to initial conditions and e<ternal pertur#ations/ F/, 4 F/,A un0avora#le systems might 0orm apprecia#le product Analy=e e;uili#rium systems;uantitatively- to e<plain the relative ;uantities o0 products and reactants @iven an initial set o0 conditions- calculate the reaction ;uotient and determine the shi0t that 'ill occur as the system proceeds to e;uili#rium Connect the calculation o0 Q to the "inetics o0 the 0or'ard and reverse reactions :erive the relationship #et'een stoichiometry and calculation o0 the e;uili#rium constant E<plain 'hy su#stances in the solid or li;uid phase are not included in the calculation o0 an e;uili#rium constant :erive !eChatelierHs Principle Apply !eChatelierHs Inquiry Lab: +pplications of LeChatelier ;rinciples /& Apply deli#erate stresses to si< di00erent e;uili#rium systems to cause the e;uili#rium to shi0t and the color to change :esign the necessary stresses on these systems to create a rain#o'4colored display %generate all colors o0 the rain#o') &ome'or" Qui==es %'ee"ly) Unit $D %!am Summative&
Curriculum Map: Advanced Placement Chemistry
Principle to e<plain e;uili#rium shi0ts o#served in the la# %color change- etc/) Quantitatively analy=e the thermodynamic 0avora#ility o0 a given chemical reaction %@o) .denti0y 2ronsted4 !o'ry acids and #ases and their conDugates :escri#e the relationship #et'een N&6>OP and N>&4P 'ith respect to J' in 'ateracidic solution- and #asic solution +elate the p& scale to the relative concentrations o0 hydronium and hydro<ide ions :erive the relationship #et'een Ja- J#- and J' to e<plain the relationship #et'een strength o0 an acid/#ase and it conDugate Use representations to e<plain and predict the acid4#ase properties o0 salt solutions
Unit ,,: Additional Aspects o0 A;ueous E;uili#rium ,/E Atoms are conserved in physical and chemical processes/ 6/A Chemical changes are represented #y a #alanced chemical e;uation that identi0ies the ratios 'ith 'hich reactants react and products 0orm/ 6/2 Chemical reactions can #e classi0ied #y considering 'hat the reactants are- 'hat the products are- or ho' they change 0rom one into the other/ Classes o0 chemical reactions include synthesisdecomposition- acid4#ase- and o<idation4reduction reactions/ F/A Chemical e;uili#rium is a dynamic- reversi#le state in 'hich rates o0 opposing processes are e;ual/
2ig .dea ,: $he chemical elements are 0undamental #uilding materials o0 matter- and all matter can #e understood in terms o0 arrangements o0 atoms/ $hese atoms retain their identity in chemical reactions/ ,/3A 2ig .dea 3: Chemical and physical properties o0 materials can #e e<plained #y the structure and the arrangement o0 atoms- ionsor molecules
Inquiry Lab: Euantitative Determination of the acid content of fruit Fuices Use titration 'ith sodium hydro<ide to determine the molar concentration o0 acids in various consumer #everages Choose an appropriate indicator 0or titration o0 acid in 0ruit Duice 'ith Na>&
!a# Port0olio E<it Passes !a# Qui= ? :etermination o0 Acid content o0 *ruit Cuices !a# Qui= ? :etermination o0 :issociation Constant o0 a Iea" Acid !a# Qui= ? Common &ousehold Products and 2u00ering Pro#lem Set ,3 &ome'or" Qui==es %'ee"ly) Unit $$ %!am Summative&
2ro'n- !eMay Chapter ,F Chapter ,8 *linn AP Advanced .n;uiry !a#s: 7,5 College 2oard AP Chemistry @uided4.n;uiry E<periments: 7- ,5
Lab: Determination of Dissociation Constant of a .eaB +cid Use titration to determine the Ja and pJa o0 an un"no'n acid Use the resulting solution 0rom the titration %endpoint) to test the properties o0 a #u00er
Inquiry Lab: 3o .hat
Curriculum Map: Advanced Placement Chemistry
F/2 Systems at e;uili#rium are responsive to e<ternal pertur#ations- 'ith the response leading to a change in the composition o0 the system/ F/C Chemical e;uili#rium plays an important role in acid4 #ase chemistry and in solu#ility/ and the 0orces #et'een them/ 3/, 3/3 2ig .dea 6: Changes in matter involve the rearrangement and/or reorgani=ation o0 atoms and/or the trans0er o0 electrons/ 6/6 6/8 2ig .dea F: Any #ond or intermolecular attraction that can #e 0ormed can #e #ro"en/ $hese t'o processes are in a dynamic competitionsensitive to initial conditions and e<ternal pertur#ations/ F/, Use molecular structure to e<plain the relative strength o0 acids and #ases Calculate the p& o0 'ea" acid and #ase solutions Analy=e acid4#ase systems ;uantitatively %including polyprotic acids) Use !eChatelierHs Principles to analy=e an e;uili#rium system ;uantitatively 'ith respect to the Common4.on E00ect Apply the Common4 .on E00ect to #u00er systems Analy=e the p& throughout titrations to identi0y the endpoint and #u00ering regions %di00erent com#inations o0 strong/'ea" acids and #ases) E<plain 'hy a #u00ering region occurs 'hen titrating acids and #ases Calculate the p& o0 a %!tent do Common 'ousehold ;roducts have Buffering +ctivity8 .nvestigate the #u00ering capacity and #u00er components o0 various consumer products @enerate a titration curve 0or a 'ea"- poly4 protic acid and identi0y the #u00ering regions 0rom the curve :esign a procedure to determine the #u00ering agents in eight di00erent household productsincluding 0ood#everages- and over4 the4counter medications Calculate pJa and analy=e #u00er capacity o0 these products
'ydrolysis of Salts2Indicator Lab
Curriculum Map: Advanced Placement Chemistry
F/3 F/9 F/,, 4 F/35 #u00er system :escri#e the e00ect o0 adding strong acid or #ase to a #u00er system at the particulate level :esign a #u00er solution 'ith a target p& and #u00er capacity #y selecting an appropriate conDugate acid4#ase pair and estimate the concentrations needed to achieve the desired capacity Analy=e a titration curve 0or a polyprotic acid Use the solu#ility4 product constant %Jsp) to descri#e the solu#ility o0 a given salt Analy=e the solu#ility and ion concentration o0 saturated solutions Use !eChatelierHs Principle to descri#e and predict the 0actors that a00ect the solu#ility o0 a salt %Common4.on e00ect- temperaturep&)
Curriculum Map: Advanced Placement Chemistry
Use Qualitative Analysis to determine the contents o0 an un"no'n solution Unit ,3: Electrochemistry 6/C Chemical and physical trans0ormations may #e o#served in several 'ays and typically involve a change in energy/ 5/2 Energy is neither created nor destroyed- #ut only trans0ormed 0rom one 0orm to another/ 5/E Chemical or physical processes are driven #y a decrease in enthalpy or an increase in entropy- or #oth/ F/A Chemical e;uili#rium is a dynamic- reversi#le state in 'hich rates o0 opposing processes are e;ual/ F/2 Systems at e;uili#rium are responsive to e<ternal pertur#ations- 'ith the response leading to a change in the composition o0 the system/ 2ig .dea 3: Chemical and physical properties o0 materials can #e e<plained #y the structure and the arrangement o0 atoms- ionsor molecules and the 0orces #et'een them/ Use the hal04reaction method to #alance redo< e;uations %Conservation o0 Mass and Conservation o0 Charge) Analy=e data regarding galvanic or electrolytic cells to identi0y properite so0 the underlying redo< reactions Lab: @edo! 3itration '()(& G +nalysis of 'ydrogen ;ero!ide :esign an e<periment to analy=e the concentration %percent composition) o0 hydrogen pero<ide through an o<idation4 reduction titration 'ith potassium permanganate Standardi=e a solution o0 potassium permanganate #y redo< titration !a# Port0olio E<it Passes !a# Qui= ? Analysis o0 &ydrogen Pero<ide Pro#lem Set ,6 &ome'or" Qui==es %'ee"ly) Unit $( %!am Summative& *linn AP Advanced .n;uiry !a#s: 9 College 2oard AP Chemistry @uided4.n;uiry E<periments: 9 2ro'n- !eMay Chapter 3A
2ased on hal04cell 3/,5 reactions- an activity series- standard 2ig .dea 6: potentials- or Changes in matter *aradayHs !a'sinvolve the students can ma"e ;ualitative or rearrangement ;uantitative and/or reorgani=ation o0 predictions a#out galvanic or electrolytic atoms and/or the reactions trans0er o0 electrons/ Calculate cell potentials under 6/,,4 6/,6 nonstandard conditions %Nernst 2ig .dea F: e;uation) Any #ond or intermolecular Apply the concepts attraction that associated 'ith voltaic
%lectroplating +ctivity
Curriculum Map: Advanced Placement Chemistry
can #e 0ormed can #e #ro"en/ $hese t'o processes are in a dynamic competitionsensitive to initial conditions and e<ternal pertur#ations/ F/3 cells %spontaneous electrochemical processes) to design strategies to com#at corrosion o0 our in0rastructure and to analy=e the 0unction o0 #atteries and 0uel cells :esign electrolytic cells that use electricity to per0orm nonspontaneous electrochemical reactions Collect and analy=e ;uantitative data 0rom voltaic and electrolytic cells
Curriculum Map: Advanced Placement Chemistry
;urpose: $he Advanced Placement Chemistry course is designed to prepare academically talented science students 0or the Advanced Placement Chemistry e<amination and to ena#le these gi0ted students to develop their science s"ills and to possi#ly receive college credit 0or Chemistry studied in high school/ .n addition the course is designed to give these students a 0ull appreciation o0 the principles o0 Chemistry through the integrated e<periences o0 la# 'or"- pro#lem solving- and class discussions/ Course content has #een structured around the #ig ideas in the curriculum 0rame'or" and should provide students 'ith a deep understanding o0 Chemistry to promote success and application in 0urther study o0 science and its applications/ HC@(I
The Six Big Ideas $& $he chemical elements are 0undamental #uilding materials o0 matter- and all matter can #e understood in terms o0 arrangements o0 atoms/ $hese atoms retain their identity in chemical reactions/ (& Chemical and physical properties o0 materials can #e e<plained #y the structure and the arrangement o0 atoms- ions- or molecules and the 0orces #et'een them/ 4& Changes in matter involve the rearrangement and/or reorgani=ation o0 atoms and/or the trans0er o0 electrons/ 5& +ates o0 chemical reactions are determined #y details o0 the molecular collisions/ 6& $he la's o0 thermodynamics descri#e the essential role o0 energy and e<plain and predict the direction o0 changes in matter/ 9& Any #ond or intermolecular attraction that can #e 0ormed can #e #ro"en/ $hese t'o processes are in a dynamic competition- sensitive to initial conditions and e<ternal pertur#ations/ Science Practices $& (& 4& 5& 6& 9& <& $he student can use representations and models to communicate scienti0ic phenomena and solve scienti0ic pro#lems/ $he student can use mathematics appropriately/ $he student can engage in scienti0ic ;uestioning to e<tend thin"ing or to guide investigations 'ithin the conte<t o0 the AP course/ $he student can plan and implement data collection strategies in relation to a particular scienti0ic ;uestion/ $he student can per0orm data analysis and evaluation o0 evidence/ $he student can 'or" 'ith scienti0ic e<planations and theories/ $he student is a#le to connect and relate "no'ledge across various scales- concepts- and representations in and across domains/
You might also like
- IB Chemistry Syllabus - Core OnlyDocument89 pagesIB Chemistry Syllabus - Core OnlyHavila SaafiNo ratings yet
- AP Biology Cell Structure and Function ReviewDocument2 pagesAP Biology Cell Structure and Function ReviewMaryam AllanaNo ratings yet
- AP Chemistry - Electrochemical Cells LabDocument6 pagesAP Chemistry - Electrochemical Cells LabJonathan Chen100% (7)
- Ap Chemistry Review SheetDocument9 pagesAp Chemistry Review Sheetapi-595413521No ratings yet
- Ap BiologyDocument11 pagesAp BiologyChandra NekkantiNo ratings yet
- Chemical Kinetics SlidesDocument87 pagesChemical Kinetics SlidesFarith AfifiNo ratings yet
- AP Chapter 13 MC Practice Questions With MC AnswersDocument9 pagesAP Chapter 13 MC Practice Questions With MC AnswersapantollanoNo ratings yet
- AP Chemistry SAMPLE 1Document88 pagesAP Chemistry SAMPLE 1Patrick CollinsNo ratings yet
- AP Biology Interdependence FRQ/Essay 1998Document3 pagesAP Biology Interdependence FRQ/Essay 1998Smart428100% (1)
- Determination of Ka of Weak AcidsDocument3 pagesDetermination of Ka of Weak Acidshdlee888100% (1)
- AP Biology SyllabusDocument2 pagesAP Biology Syllabusbiologyfreak132No ratings yet
- AP Chem Practice TestDocument14 pagesAP Chem Practice Testamrdeck1No ratings yet
- AP Biology Enzyme Lab ReportDocument3 pagesAP Biology Enzyme Lab ReportPatrick100% (6)
- AP Physics Lab GuideDocument75 pagesAP Physics Lab GuidejdlawlisNo ratings yet
- Syllabus OutlineDocument2 pagesSyllabus Outlineapi-246410374No ratings yet
- Weak Acid and Base Equilibrium ReviewDocument20 pagesWeak Acid and Base Equilibrium Review任思诗No ratings yet
- AP Chemistry FR Test BankDocument7 pagesAP Chemistry FR Test BankzeustamNo ratings yet
- Chemical Bonding - Study NotesDocument15 pagesChemical Bonding - Study NotesTamoghna DeyNo ratings yet
- AP Chem CH 6 Practice Quiz Heat Capacity ReactionsDocument3 pagesAP Chem CH 6 Practice Quiz Heat Capacity Reactionsprin ppNo ratings yet
- Practice Exam 4Document7 pagesPractice Exam 4Hasantha PereraNo ratings yet
- AP Chemistry Summer AssignmentDocument6 pagesAP Chemistry Summer AssignmentDavina MarstonNo ratings yet
- IB Biology 3ED PEQ AnswersDocument110 pagesIB Biology 3ED PEQ AnswersGeorge KoukouvesNo ratings yet
- C1 - Basic Concepts of Chemistry - Solutions (v18) - HD - CLDocument20 pagesC1 - Basic Concepts of Chemistry - Solutions (v18) - HD - CLAashish DubeyNo ratings yet
- C 1 2 2025 Topic Test MsDocument4 pagesC 1 2 2025 Topic Test MsRawanMazen SharifNo ratings yet
- HHMI AP Biology GuideDocument66 pagesHHMI AP Biology Guidecharanmann9165No ratings yet
- Chapter 19 Ap Chemistry OutlineDocument9 pagesChapter 19 Ap Chemistry OutlineElba MartinesNo ratings yet
- AP Chem CH 3 Practice QuizAP Chemistry Practice TestDocument5 pagesAP Chem CH 3 Practice QuizAP Chemistry Practice TesthydrocrackermanNo ratings yet
- Halogenoalkanes and Alcohols HWDocument13 pagesHalogenoalkanes and Alcohols HWapi-504683923No ratings yet
- Apch3.1 Problems-Equilibrium AnsDocument5 pagesApch3.1 Problems-Equilibrium AnsQueenQiNo ratings yet
- AP Environmental Science - Course Syllabus-2Document4 pagesAP Environmental Science - Course Syllabus-2nathan6limNo ratings yet
- 5.2 Introduction To Rate Law StudentDocument6 pages5.2 Introduction To Rate Law StudentSyed RazaNo ratings yet
- 7 CIE IGCSE Additional Mathematics Topical Past Paper Logarithmic and Exponential Functions PDFDocument15 pages7 CIE IGCSE Additional Mathematics Topical Past Paper Logarithmic and Exponential Functions PDFChloe CxyNo ratings yet
- What Is A Mole SummativeDocument8 pagesWhat Is A Mole Summativeapi-291560513No ratings yet
- Chapter 4 Chemical Bonding and Molecular StructureDocument26 pagesChapter 4 Chemical Bonding and Molecular StructureYash PlayNo ratings yet
- Ap Chem - Chapter 1 Reading GuideDocument21 pagesAp Chem - Chapter 1 Reading Guideapi-475547739No ratings yet
- Caffeine Extraction 1 PDFDocument25 pagesCaffeine Extraction 1 PDFShanay ShahNo ratings yet
- Physics Grade 10 Physics Ch7 Geometrical OpticsDocument62 pagesPhysics Grade 10 Physics Ch7 Geometrical Opticsapi-19999615No ratings yet
- H2 Bio Paper 2 Glycogen and Transport ProteinsDocument10 pagesH2 Bio Paper 2 Glycogen and Transport ProteinsSamuel TeohNo ratings yet
- Atomic Structure (AP MC)Document4 pagesAtomic Structure (AP MC)Nyxas IoannisNo ratings yet
- AP Biology Survival Guide 10Document4 pagesAP Biology Survival Guide 10Mo AlexandriaNo ratings yet
- Unit 1 TestDocument5 pagesUnit 1 Testapi-485795043No ratings yet
- DP Math: G12 SL Function Transformation Review: 1a. (2 Marks) The Diagram Below Shows The GraphDocument1 pageDP Math: G12 SL Function Transformation Review: 1a. (2 Marks) The Diagram Below Shows The GraphAlex SteinkampNo ratings yet
- AP Chemistry Study Guide: Chapter 14: Acids and Bases and Chapter 15, 16.1 and 21.3: Aqueous and Acid-Base EquilibriaDocument8 pagesAP Chemistry Study Guide: Chapter 14: Acids and Bases and Chapter 15, 16.1 and 21.3: Aqueous and Acid-Base Equilibrialorraine_cuaNo ratings yet
- IB BIOLOGY SL TOPIC 3 Nucleic Acids & Proteins and Chemical Elements & WaterDocument13 pagesIB BIOLOGY SL TOPIC 3 Nucleic Acids & Proteins and Chemical Elements & WaterweeNo ratings yet
- Packet Unit 1Document36 pagesPacket Unit 1Amelia Simmons0% (1)
- Chemical Concentration and MolarityDocument7 pagesChemical Concentration and Molarityapi-352917620No ratings yet
- TB Final2023 643ef57734b858.643ef57a5a0fb6.40844090Document71 pagesTB Final2023 643ef57734b858.643ef57a5a0fb6.40844090Sameh NoorNo ratings yet
- B1.1 - Carbohydrates and LipidsDocument4 pagesB1.1 - Carbohydrates and LipidslittleianlauNo ratings yet
- 5B - Stoichiometry 2Document41 pages5B - Stoichiometry 2Vimanan A/L S. VelangganiNo ratings yet
- C 1 1 2025 Topic Test MsDocument4 pagesC 1 1 2025 Topic Test MsRawanMazen SharifNo ratings yet
- Kami Export - Benjamin Ratin - 1.6 Photoelectron Spectroscopy StudentDocument3 pagesKami Export - Benjamin Ratin - 1.6 Photoelectron Spectroscopy StudentBenjamin RatinNo ratings yet
- AP Physics C Mechanics Sample Syllabus 1Document9 pagesAP Physics C Mechanics Sample Syllabus 1mahmoudNo ratings yet
- AP Chem Ch 5 Practice Quiz and KeyDocument5 pagesAP Chem Ch 5 Practice Quiz and KeyhydrocrackermanNo ratings yet
- Gas Laws I SP 1617 (PreAP)Document3 pagesGas Laws I SP 1617 (PreAP)Nikhil Singh100% (1)
- Angular MotionDocument21 pagesAngular Motionsuperman618No ratings yet
- AP Chem Acids/Bases Worksheet PacketDocument5 pagesAP Chem Acids/Bases Worksheet PacketBobWilliamsNo ratings yet
- Pre Assessment Phys Behavior or MatterDocument1 pagePre Assessment Phys Behavior or Matterapi-249441006No ratings yet
- Pre Assessment Electricity Physics HDocument2 pagesPre Assessment Electricity Physics Hapi-249441006No ratings yet
- Exit Pass Post-Lab Chem Honors 1Document2 pagesExit Pass Post-Lab Chem Honors 1api-249441006No ratings yet
- Study Guide Momentum Impulse PendulumsDocument2 pagesStudy Guide Momentum Impulse Pendulumsapi-249441006No ratings yet
- Regents Review ScheduleDocument4 pagesRegents Review Scheduleapi-249441006No ratings yet
- Regents Review ScheduleDocument4 pagesRegents Review Scheduleapi-249441006No ratings yet
- Free Fall Notes 2Document3 pagesFree Fall Notes 2api-249441006No ratings yet
- Ap Chemistry Review Schedule 2014Document2 pagesAp Chemistry Review Schedule 2014api-249441006No ratings yet
- Choice Exit PassDocument1 pageChoice Exit Passapi-249441006No ratings yet
- Horizontally Launched Projectile Lab 11 2013Document2 pagesHorizontally Launched Projectile Lab 11 2013api-249441006No ratings yet
- Re Write Procedures 10 12Document1 pageRe Write Procedures 10 12api-249441006No ratings yet
- Recycling ProjectDocument2 pagesRecycling Projectapi-249441006No ratings yet
- Lab Stoichiometry Iron With Copper II SulfateDocument5 pagesLab Stoichiometry Iron With Copper II Sulfateapi-249441006No ratings yet
- Study Guide RedoxDocument2 pagesStudy Guide Redoxapi-249441006No ratings yet
- Ap Chemistry Final Project 2013Document7 pagesAp Chemistry Final Project 2013api-249441006No ratings yet
- Lab Stoichiometry Iron With Copper II SulfateDocument5 pagesLab Stoichiometry Iron With Copper II Sulfateapi-249441006No ratings yet
- Molecular Geometry Lab 12 6 12 Tenure PortfolioDocument5 pagesMolecular Geometry Lab 12 6 12 Tenure Portfolioapi-249441006No ratings yet
- Environmental Curriculum MapDocument12 pagesEnvironmental Curriculum Mapapi-249441006No ratings yet
- Reading Assignment Nuclear ChemistryDocument2 pagesReading Assignment Nuclear Chemistryapi-249441006No ratings yet
- P V T Relationships Tenure PortfolioDocument4 pagesP V T Relationships Tenure Portfolioapi-249441006No ratings yet
- Obhs Chem Scope and SequenceDocument13 pagesObhs Chem Scope and Sequenceapi-249441006No ratings yet
- Video Notes Molecular PolarityDocument4 pagesVideo Notes Molecular Polarityapi-249441006No ratings yet
- Testing Predictions About Proj MotionDocument4 pagesTesting Predictions About Proj Motionapi-249441006No ratings yet
- Flexible GroupingDocument2 pagesFlexible Groupingapi-249441006No ratings yet
- Notes Coulombs Law VideosDocument2 pagesNotes Coulombs Law Videosapi-249441006No ratings yet
- Mu of The Shoe InquiryDocument3 pagesMu of The Shoe Inquiryapi-249441006No ratings yet
- Annicelli Lesson Plan 12 16 13Document4 pagesAnnicelli Lesson Plan 12 16 13api-249441006No ratings yet
- Annicelli Lesson Plan 11 16 12Document3 pagesAnnicelli Lesson Plan 11 16 12api-249441006No ratings yet
- Acid Base Properties of Salts PortfolioDocument3 pagesAcid Base Properties of Salts Portfolioapi-249441006No ratings yet
- Maporillal Vs DeliciasDocument14 pagesMaporillal Vs DeliciasJessica RiveraNo ratings yet
- Microelectronics: Circuit Analysis and Design, 4 Edition by D. A. Neamen Problem SolutionsDocument6 pagesMicroelectronics: Circuit Analysis and Design, 4 Edition by D. A. Neamen Problem SolutionsJano Jesus AlexNo ratings yet
- Fire Strength Performance ofDocument3 pagesFire Strength Performance ofMarimuthu KannimuthuNo ratings yet
- Operations Management and Decision MakingDocument55 pagesOperations Management and Decision MakingAnkit SinghNo ratings yet
- Primary Reformer TubesDocument10 pagesPrimary Reformer TubesAhmed ELmlahyNo ratings yet
- Service Repair Manual - (Cat) Caterpillar 3126 Machine Engine SN 1bw, 55kDocument1,094 pagesService Repair Manual - (Cat) Caterpillar 3126 Machine Engine SN 1bw, 55kLen Wal100% (2)
- Assignment No.3 Bolted JointsDocument6 pagesAssignment No.3 Bolted JointsYash SahuNo ratings yet
- SQL Injection Attack Detection and Preve PDFDocument12 pagesSQL Injection Attack Detection and Preve PDFPramono PramonoNo ratings yet
- EC424 Monetary Economics (Michaelmas Term) Additional QuestionsDocument5 pagesEC424 Monetary Economics (Michaelmas Term) Additional QuestionsSteamPunkNo ratings yet
- Quizlet-Philippine Electrical CodeDocument2 pagesQuizlet-Philippine Electrical Codena zafira0% (1)
- Recent Developments in Ultrasonic NDT Modelling in CIVADocument7 pagesRecent Developments in Ultrasonic NDT Modelling in CIVAcal2_uniNo ratings yet
- Adobe After Effects CS3 Keyboard Shortcuts GuideDocument14 pagesAdobe After Effects CS3 Keyboard Shortcuts GuideBrandon Sirota100% (1)
- DMTH505 Measure Theorey and Functional Analysis PDFDocument349 pagesDMTH505 Measure Theorey and Functional Analysis PDFJahir Uddin LaskarNo ratings yet
- h2 PR Final Key SP 13Document3 pagesh2 PR Final Key SP 13George ConstantinouNo ratings yet
- Hospital Management System: A Project Report OnDocument24 pagesHospital Management System: A Project Report OnRama GayariNo ratings yet
- Name Source Description Syntax Par, Frequency, Basis)Document12 pagesName Source Description Syntax Par, Frequency, Basis)alsaban_7No ratings yet
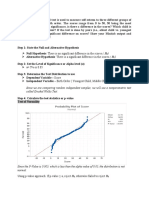
- Step 1: State The Null and Alternative HypothesisDocument3 pagesStep 1: State The Null and Alternative HypothesisChristine Joyce BascoNo ratings yet
- SCADADocument14 pagesSCADANunna BaskarNo ratings yet
- Cs8080 - Irt - Notes AllDocument281 pagesCs8080 - Irt - Notes Allmukeshmsd2No ratings yet
- Schindler Drive Chain MaintenanceDocument9 pagesSchindler Drive Chain MaintenanceKevin aliNo ratings yet
- TONISITASDocument17 pagesTONISITASDewi Ria ONo ratings yet
- Common MisconceptionsDocument7 pagesCommon MisconceptionsBoazz750No ratings yet
- Ace Signal and System PDFDocument144 pagesAce Signal and System PDFYash Rai100% (1)
- Propeller Model Tests GuideDocument12 pagesPropeller Model Tests GuideOdpewğw RlelelwNo ratings yet
- VPRS-4300D Catalogue PDFDocument4 pagesVPRS-4300D Catalogue PDFHoàngTrầnNo ratings yet
- A-019730-1647416754604-137865-W.M.Supun Anjana DSADocument175 pagesA-019730-1647416754604-137865-W.M.Supun Anjana DSADishan SanjayaNo ratings yet
- Non-Performing Assets: A Comparative Study Ofsbi&Icici Bank From 2014-2017Document8 pagesNon-Performing Assets: A Comparative Study Ofsbi&Icici Bank From 2014-2017Shubham RautNo ratings yet
- Business Calculus NotesDocument38 pagesBusiness Calculus NotesTom KowalskiNo ratings yet
- Flex-Shaft Attachment Instructions Model 225: WarningDocument1 pageFlex-Shaft Attachment Instructions Model 225: WarningFernando Lopez Lago100% (1)