Professional Documents
Culture Documents
A2 Final
Uploaded by
harshanauoc0 ratings0% found this document useful (0 votes)
26 views24 pagesfinal paper
Original Title
a2 final
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Documentfinal paper
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF or read online from Scribd
0 ratings0% found this document useful (0 votes)
26 views24 pagesA2 Final
Uploaded by
harshanauocfinal paper
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF or read online from Scribd
You are on page 1of 24
(@) (i) State what is meant by the internal energy of a system.
2]
(ii) Explain why, for an ideal gas, the internal energy is equal to the total kinetic energy
of the molecules of the gas.
[2]
(b) The mean kinetic energy of a molecule of an ideal gas is given by the expression
where k is the Boltzmann constant and T is the thermodynamic temperature of the gas.
A cylinder contains 1.0mol of an ideal gas. The gas is heated so that its temperature
changes from 280K to 40K.
Calculate the change in total kinetic energy of the gas molecules.
change in energy = ae. J
(ii) During the heating, the gas expands, doing 1.5 x 10° J of work.
State the first law of thermodynamics. Use the law and your answer in (i) to
determine the total energy supplied to the gas.
(a) Define electric potential at a point.
2
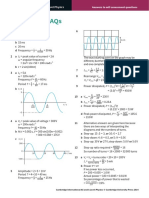
(b) Two point charges A and B are separated by a distance of 20nm in a vacuum, as
illustrated in Fig. 3.1
20nm
Fig. 3.1
A point P is a distance x from A along the line AB.
The variation with distance x of the electric potential V, due to charge A alone is shown
in Fig. 3.2,
0.8
ntial
0.6
04
0.2
2 4 6 8 10 12 4 16
xinm
Fig. 3.2
The variation with distance x of the electric potential V due to charge B alone is also
shown in Fig. 3.2
@
(ii)
(iii)
(iv)
State and explain whether the charges A and B are of the same, or opposite, sign.
a)
By reference to Fig, 3.2, state how the combined electric potential due to both
charges may be determined,
af]
Without any calculation, use Fig. 3.2 to estimate the distance x at which the
combined electric potential of the two charges is a minimum.
x nm [1]
The point P is a distance x = 10nm from A.
‘An e-particle has kinetic energy E,, when at infinity.
Use Fig. 3.2 to determine the minimum value of E, such that the a-particle may
travel from infinity to point P.
J 1]
You might also like
- UnitDocument8 pagesUnitharshanauocNo ratings yet
- 2017 p1Document14 pages2017 p1harshanauocNo ratings yet
- A CarDocument6 pagesA CarharshanauocNo ratings yet
- Answers To EOC Questions: Cambridge International A Level PhysicsDocument2 pagesAnswers To EOC Questions: Cambridge International A Level PhysicsharshanauocNo ratings yet
- Answers To EOC Questions: Cambridge International AS Level PhysicsDocument2 pagesAnswers To EOC Questions: Cambridge International AS Level PhysicsharshanauocNo ratings yet
- Harshana Perera (B.SC - BIT, SCJP) 2016 Paper 1Document15 pagesHarshana Perera (B.SC - BIT, SCJP) 2016 Paper 1harshanauocNo ratings yet
- Answers To Saqs: Cambridge International A Level PhysicsDocument2 pagesAnswers To Saqs: Cambridge International A Level Physicsharshanauoc100% (1)
- EOCQ Ans 19Document2 pagesEOCQ Ans 19harshanauocNo ratings yet
- Answers To Saqs: Cambridge International A Level PhysicsDocument2 pagesAnswers To Saqs: Cambridge International A Level PhysicsharshanauocNo ratings yet
- Cambridge International AS Level Physics Answers to SAQs Chapter 14Document2 pagesCambridge International AS Level Physics Answers to SAQs Chapter 14harshanauoc100% (1)
- Answers To EOC Questions: Cambridge International AS Level PhysicsDocument2 pagesAnswers To EOC Questions: Cambridge International AS Level PhysicsharshanauocNo ratings yet
- EOCQ Ans 15Document1 pageEOCQ Ans 15harshanauoc50% (2)
- SAQ Ans 3Document2 pagesSAQ Ans 3harshanauocNo ratings yet
- W MCQDocument8 pagesW MCQharshanauocNo ratings yet
- EOCQ Ans 22Document2 pagesEOCQ Ans 22harshanauocNo ratings yet
- SAQ Ans 10Document2 pagesSAQ Ans 10harshanauoc0% (1)
- Answers To EOC Questions: Cambridge International A Level PhysicsDocument2 pagesAnswers To EOC Questions: Cambridge International A Level Physicsharshanauoc100% (1)
- Answers To Saqs: Cambridge International As Level PhysicsDocument2 pagesAnswers To Saqs: Cambridge International As Level PhysicsharshanauocNo ratings yet
- Answers To Saqs: Cambridge International A Level PhysicsDocument1 pageAnswers To Saqs: Cambridge International A Level PhysicsharshanauocNo ratings yet
- Answers To Saqs: Cambridge International As Level PhysicsDocument2 pagesAnswers To Saqs: Cambridge International As Level PhysicsharshanauocNo ratings yet
- Answers To Saqs: Cambridge International As Level PhysicsDocument2 pagesAnswers To Saqs: Cambridge International As Level Physicsharshanauoc100% (3)
- SAQ Ans 6Document3 pagesSAQ Ans 6harshanauocNo ratings yet
- SAQ Ans 28Document2 pagesSAQ Ans 28harshanauocNo ratings yet
- A Level Physics answers to self-assessment questionsDocument2 pagesA Level Physics answers to self-assessment questionsharshanauocNo ratings yet
- SAQ Ans 24Document2 pagesSAQ Ans 24harshanauocNo ratings yet
- SAQ Ans 32Document2 pagesSAQ Ans 32harshanauocNo ratings yet
- Answers To Saqs: Cambridge International A Level PhysicsDocument1 pageAnswers To Saqs: Cambridge International A Level PhysicsharshanauocNo ratings yet
- SAQ Ans 7Document2 pagesSAQ Ans 7harshanauocNo ratings yet
- SAQ Ans 6Document3 pagesSAQ Ans 6harshanauocNo ratings yet
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5783)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (890)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (72)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)