Professional Documents
Culture Documents
Aem00179 0232
Uploaded by
Huy NgoOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Aem00179 0232
Uploaded by
Huy NgoCopyright:
Available Formats
APPLIED AND ENVIRONMENTAL MICROBIOLOGY, OCt. 1982, p. 990-991 0099-2240/82/100990-02$02.
00/0 Copyright 1982, American Society for Microbiology
Vol. 44, No. 4
Improved Medium for Isolation of Azospirillum
Aires, Argentina
Received 6 January 1982/Accepted 28 June 1982
spp.
ENRIQUE A. RODRIGUEZ CACERES Departamento de Microbiologia, Instituto Nacional de Tecnologia, Agropecuaria, 1712 Castelar, Buenos
Colonies of Azospirillum spp. could be readily distinguished from colonies of other diazotrophs by scarlet coloration in culture media in which Congo red was included.
Azospirillum spp. are among the most important bacteria involved in N2 fixation in grasses. It is normal for Azospirillum spp. to be isolated from nitrogen-free culture media inoculated with soil or roots. However, Dobereiner et al. (2) have reported that isolation of pure cultures from samples collected in temperate regions is difficult. Vlassak and Reynders (6) have found that in many cases, nitrogen-fixing bacteria are closely associated with other microorganisms and that obtaining pure isolates is not easy. The use of Congo red for distinguishing between rhizobia and other bacteria is well known (3). This paper describes a simple technique which permits the recognition of Azospirillum colonies on plates and facilitates the isolation of pure cultures. Two media were employed: nitrogen-free semisolid malate (NFb) enrichment medium (1), and Rojo Congo (Congo red; RC), an isolation medium. On the basis of the chemical composition of medium 79 (3), several carbon sources for Azospirillum spp. and concentrations of yeast extract (0 to 1 g * liter-) were used in RC medium, which also contained the following (grams per liter of distilled water): K2HPO4, 0.5; MgSO4 - 7H20, 0.2; NaCl, 0.1; yeast extract, 0.5; FeCl3 * 6H20, 0.015; DL-malic acid, 5; KOH, 4.8; and agar, 20. The pH was adjusted to 7.0 with 0.1 N KOH, and the medium was autoclaved at 121C for 20 min. I added 15 ml of a 1:400 aqueous solution of Congo red (autoclaved separately) aseptically to each liter of the melted medium just before tubing or use. Samples of soil from corn (Zea mays L.), sorghum (Sorghum sp.), and rice (Oryza sativa L.) crops, collected in areas of Argentina, or root pieces from the same species (washed with sterile water or treated with 1% chloramine T [4]) were placed into tubes, each of which contained 5 ml of NFb medium. These enrichment cultures were incubated at 37C for 72 h. A white, dense, undulating, diffuse pellicle was
FIG. 1. Isolation plates of RC medium. Incubation was at 37C for 72 h. Azospirillum colonies were easily recognized.
then observed 1 to 4 mm below the surface. Examination of wet mounts by phase-contrast microscopy revealed, among other microorganisms, rods with fat droplets and active movements characteristic of Azospirillum spp. Posi-
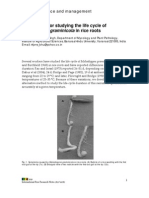
FIG. 2. First streaks showing small Azospirillum colonies among the contaminants.
990
VOL . 44, 1982
NOTES
991
FIG. 3. Azospirillum colonies after 96 h of incubation. Magnification, x 10.
tive cultures were serially diluted 10-fold in sterile tap water to 10-4 to 10-5. Loopfuls of the dilutions were streaked on plates of RC medium, which were incubated at 37C for 72 h. Light-pink and colorless colonies were observed after 48 h. After 72 h, the light-pink colonies became scarlet (Fig. 1). Small scarlet colonies were observed in the first streaks, indicating the presence of Azospirillum spp. among the contaminants (Fig. 2). Phase-contrast microscopic examination of wet mounts of the scarlet colonies revealed rods resembling Azospirillum cells. The colonies were diluted in sterile tap water, and the dilutions were streaked on plates of RC medium to check the purity of the isolates. Uniformity of colony color was observed. Diagnostic biochemical and physiological tests were conducted by the method of Tarrand et al. (5) and confirmed the identity of the strains. To confirm the usefulness of this technique, I streaked a loopful of an Azospirillum brasilense culture from the American Type Culture Collection (ATCC 29145) on RC medium. All colonies became scarlet after incubation. Two species from roots and soil samples were
identified on the basis of the capacity to use glucose as the sole carbon source for growth in NFb medium containing biotin (5): Azospirillum lipoferum and A. brasilense. Scarlet colonies of both species grew in plates of this medium. Colonies that developed in petri dishes of RC medium incubated at 37C for 96 h had the following characteristics: scarlet color, abundant growth, dry consistency, diameter of 1.5 to 2 mm, round or irregular form, undulate edge, and rugose surface with ridges radiating from the center (Fig. 3). The colonies of other rootassociated bacteria (species of Bacillus, Enterobacter, Klebsiella and Pseudomonas) are circular, convex, translucent, and smooth, have an entire margin, and do not absorb Congo red. Many pure cultures were readily obtained by this method.
LITERATURE CITED 1. Baldani, V. L. D., and J. Dfbereiner. 1980. Host-plant specificity in the infection of cereals with Azospirillum spp. Soil Biol. Biochem. 12:433-439. 2. D6bereiner, J., I. E. Marriel, and M. Nery. 1976. Ecological distribution of Spirillum lipoferum Beijerinck. Can. J. Microbiol. 22:1464-1473. 3. Fred, E. B., and S. A. Waksman. 1928. Laboratory manual of general microbiology, p. 33 and 96. McGraw-Hill Book Co., New York. 4. Patrlquin, D. G., and J. Dobereiner. 1978. Light microscopy observations of tetrazolium-reducing bacteria in the endorhizosphere of maize and other grasses in Brazil. Can. J. Microbiol. 24:734-742. 5. Tarrand, J., N. R. Krieg, and J. Dobereiner. 1978. A taxonomic study of the Spirillum lipoferum group, with descriptions of a new genus, Azospirillum gen. nov. and two species, Azospirillum lipoferum (Beijerinck) comb. nov. and Azospirillum brasilense sp. nov. Can. J. Microbiol. 24:967-980. 6. Vlassak, K., and L. Reynders. 1978. Associative dinitrogen fixation in temperate regions, p. 71. In Isotopes in biological dinitrogen fixation (Proceedings of the Advisory Group, Vienna, November 1977). International Atomic Energy Agency, Vienna.
You might also like
- Atomic Structure WorksheetsDocument21 pagesAtomic Structure Worksheetssantoshkumarsir7706100% (1)
- Somatic Psychology Linda Hartley Review PDFDocument8 pagesSomatic Psychology Linda Hartley Review PDFAndres SanabriaNo ratings yet
- Fitness RX For Women - April 2014Document116 pagesFitness RX For Women - April 2014Ricardo Soares100% (2)
- Group 9 Caught in Between Modern and Contemporary ArtDocument12 pagesGroup 9 Caught in Between Modern and Contemporary Artlen lenNo ratings yet
- The Wankel Engine Design Development AppDocument271 pagesThe Wankel Engine Design Development AppFurqanNo ratings yet
- Timber Deck CargoesDocument12 pagesTimber Deck CargoeschristieSINo ratings yet
- Tillandsia Plant Harbors Nitrogen-Fixing BacteriumDocument3 pagesTillandsia Plant Harbors Nitrogen-Fixing BacteriumRichar Manuel Simanca FontalvoNo ratings yet
- Inoculant For BiofertilizersDocument15 pagesInoculant For BiofertilizersBrij Mohan SinghNo ratings yet
- Cumene ManufactringDocument74 pagesCumene ManufactringTan JieSheng100% (1)
- Isolation and Characterization of Plant Growth-Promoting RhizobacteriaDocument15 pagesIsolation and Characterization of Plant Growth-Promoting RhizobacteriaKuruni NagarajNo ratings yet
- 2012 Chandra JPPRDocument6 pages2012 Chandra JPPRknchandu1No ratings yet
- Characterization and Identification of Phyllospheric Bacterial Isolates of Rice (Oryza Sativa)Document6 pagesCharacterization and Identification of Phyllospheric Bacterial Isolates of Rice (Oryza Sativa)Dr Amrit Kumar JhaNo ratings yet
- Occurrence and Microbiological Characteristics of Azospirillum Strains Associated With Wheat Rhizoplane and EndorhizosphereDocument8 pagesOccurrence and Microbiological Characteristics of Azospirillum Strains Associated With Wheat Rhizoplane and EndorhizosphereAnantha Rama ANo ratings yet
- Phaseolus Vulgarisseed-Borne Endophytic Community With Novel Bacterial Species Such As Rhizobium Endophyticum Sp. Nov.Document6 pagesPhaseolus Vulgarisseed-Borne Endophytic Community With Novel Bacterial Species Such As Rhizobium Endophyticum Sp. Nov.lesousa454No ratings yet
- Aflatoxins Lab 1Document10 pagesAflatoxins Lab 1Thoriso Reginald MokgatleNo ratings yet
- Project 1 Micro.-2Document7 pagesProject 1 Micro.-2NkosinathiNo ratings yet
- Detection of Alternaria radicina and A. dauci from Imported Carrot SeedDocument7 pagesDetection of Alternaria radicina and A. dauci from Imported Carrot SeedPaula Ordenes DomínguezNo ratings yet
- DobereinerDocument2 pagesDobereinerJuan Jose CamachoNo ratings yet
- Marine Bacteria From Coral MucusDocument7 pagesMarine Bacteria From Coral MucusYossi CohenNo ratings yet
- Estimating Bacterial Populations on Plant Leaves Using Culture MethodsDocument6 pagesEstimating Bacterial Populations on Plant Leaves Using Culture MethodsGhazala HaqueNo ratings yet
- Clostridium Hungatei Sp. Nov., A Mesophilic, N: - Fixing Cellulolytic Bacterium Isolated From SoilDocument10 pagesClostridium Hungatei Sp. Nov., A Mesophilic, N: - Fixing Cellulolytic Bacterium Isolated From SoilRajapriya Prabha KaranNo ratings yet
- Bacterial Colonization: Growth and Competition Affect Nodulation and Root by Rhizobium MelilotiDocument5 pagesBacterial Colonization: Growth and Competition Affect Nodulation and Root by Rhizobium MelilotiALNo ratings yet
- Technique Detection Spongospora in Soil 1983 SzeDocument2 pagesTechnique Detection Spongospora in Soil 1983 SzeDario BaronaNo ratings yet
- 11chapter 3Document22 pages11chapter 3PatilNo ratings yet
- Bokdan 1977Document80 pagesBokdan 1977CarolineVasconcelosNo ratings yet
- Seasonal Variations of Bacillus Isolated From The Rhizosphere of Elaeagnus Angustifolia LDocument10 pagesSeasonal Variations of Bacillus Isolated From The Rhizosphere of Elaeagnus Angustifolia LMuthu KumarNo ratings yet
- Isolation and Characterization of Ralstonia Solanacearum Causing Bacterial Wilt of Tomato in NigeriaDocument10 pagesIsolation and Characterization of Ralstonia Solanacearum Causing Bacterial Wilt of Tomato in NigeriaElvis GergesNo ratings yet
- Caracterizarea Actiunii Larvicide La Tanta A Tulpinii de Bacillus Thurigiensis Izolat Din Sol in India PDFDocument3 pagesCaracterizarea Actiunii Larvicide La Tanta A Tulpinii de Bacillus Thurigiensis Izolat Din Sol in India PDFComan GigiNo ratings yet
- Biology of LifeDocument7 pagesBiology of LifeDrizzy MarkNo ratings yet
- Isolation and Identification of Endophytic Bacterial Strains from Sugarcane StalksDocument5 pagesIsolation and Identification of Endophytic Bacterial Strains from Sugarcane StalksMaricel EspitiaNo ratings yet
- Sources of Microbial Contamination in TC LabDocument6 pagesSources of Microbial Contamination in TC LabLau Shin YeeNo ratings yet
- What is RhizobiumDocument23 pagesWhat is RhizobiumPrasun bairagiNo ratings yet
- Endofitos en GramineasDocument4 pagesEndofitos en GramineasGregorio AroneNo ratings yet
- Morphological and Molecular Variability of Podosphaera Pannosa Causing Rose Powdery Mildew in Himachal Pradesh, IndiaDocument6 pagesMorphological and Molecular Variability of Podosphaera Pannosa Causing Rose Powdery Mildew in Himachal Pradesh, IndiavijayNo ratings yet
- v09 2 11 p98 105Document8 pagesv09 2 11 p98 105mustafi28No ratings yet
- Isolation, Identification and Characterization of Phyllosphere Fungi From VegetablesDocument6 pagesIsolation, Identification and Characterization of Phyllosphere Fungi From VegetablesInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Bacterial and Viral Pathogens AssociatedDocument10 pagesBacterial and Viral Pathogens Associatednanochabow537No ratings yet
- tmp1B25 TMPDocument12 pagestmp1B25 TMPFrontiersNo ratings yet
- Isolation and Characterization of Phosphate Solubilizing Microorganism (PSM) From The Rhizosphere and Roots of Crops Indigenous To Ihiagwa-Owerri Imo State NigeriaDocument7 pagesIsolation and Characterization of Phosphate Solubilizing Microorganism (PSM) From The Rhizosphere and Roots of Crops Indigenous To Ihiagwa-Owerri Imo State NigeriaInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Etesami PGPR RICEDocument13 pagesEtesami PGPR RICEMohammad Mosharraf HossainNo ratings yet
- Characterisation of Rhizosphere Soil Bacteria From Rice Varieties Grown Under Greenhouse EnvironmentDocument5 pagesCharacterisation of Rhizosphere Soil Bacteria From Rice Varieties Grown Under Greenhouse EnvironmentInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Morpho-Anatomical and Phylogenetic Studies of RussDocument13 pagesMorpho-Anatomical and Phylogenetic Studies of Russgeoheritage.sbicapsNo ratings yet
- Plant Growth Substances Produced by Micro-Organisms Soil and RhizosphereDocument9 pagesPlant Growth Substances Produced by Micro-Organisms Soil and RhizosphereLAURA DANIELA VALDERRAMA TORRESNo ratings yet
- Abundance of T4 Bacteriophages in Wastewater & SewageDocument4 pagesAbundance of T4 Bacteriophages in Wastewater & SewagemarychuiyNo ratings yet
- 54-Widhi Histochemical AcidsDocument10 pages54-Widhi Histochemical AcidsThiruselvam B PositiveNo ratings yet
- Microspore-Derived Embryos From Quercus Suber Anthers Mimic Zygotic Embryos and Maintain Haploidy in Long-Term Anther CultureDocument8 pagesMicrospore-Derived Embryos From Quercus Suber Anthers Mimic Zygotic Embryos and Maintain Haploidy in Long-Term Anther CultureKristem KertzeifNo ratings yet
- Carbon Va NitoDocument11 pagesCarbon Va NitoHoang AnhNo ratings yet
- ISOLATION AND N2 FIXATIONDocument8 pagesISOLATION AND N2 FIXATIONIsaac AbadNo ratings yet
- TMP EC3Document10 pagesTMP EC3FrontiersNo ratings yet
- Isolation Actinomycetes ANDRIANODocument7 pagesIsolation Actinomycetes ANDRIANOBerenice AndrianoNo ratings yet
- Stemphylium Leaf Spot of Parsley in California Caused by Stemphylium VesicariumDocument8 pagesStemphylium Leaf Spot of Parsley in California Caused by Stemphylium VesicariumJohan Sebastián Ríos ZNo ratings yet
- Brassica Oleracea Capitata L. Pratylenchus PenetransDocument7 pagesBrassica Oleracea Capitata L. Pratylenchus PenetransHalimah SiregarNo ratings yet
- Screening and Isolation of Cyclosporine-Related Compound Producing Soil Fungi From The Western Ghats, Tamil NaduDocument2 pagesScreening and Isolation of Cyclosporine-Related Compound Producing Soil Fungi From The Western Ghats, Tamil Nadusimanta86100% (1)
- Pared BacteriasDocument18 pagesPared BacteriasenadesNo ratings yet
- Sydney-Mae Low - Lab Report 1Document13 pagesSydney-Mae Low - Lab Report 1Sydney-Mae LowNo ratings yet
- Joe Rob BinsDocument4 pagesJoe Rob BinsKatie RobbinsNo ratings yet
- Isolation of Soil Mycoflora From AgriculDocument4 pagesIsolation of Soil Mycoflora From AgriculnikunjNo ratings yet
- Bacterial Blight of Rice - Isolation Identification Characterization PreservationDocument5 pagesBacterial Blight of Rice - Isolation Identification Characterization PreservationAshish GhimireNo ratings yet
- Isolation and Biochemical Identification of Soil Bacteria From North Eastern Hill University, ShillongDocument5 pagesIsolation and Biochemical Identification of Soil Bacteria From North Eastern Hill University, ShillongInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- A Study On The Isolation of Protoplasts From Mesophyll Cells of Dendrobium Queen PinkDocument5 pagesA Study On The Isolation of Protoplasts From Mesophyll Cells of Dendrobium Queen Pinkkahkashan kazmiNo ratings yet
- Intracellular bacterium infecting Acanthamoeba proposed as new genusDocument10 pagesIntracellular bacterium infecting Acanthamoeba proposed as new genussomasushmaNo ratings yet
- Identification and Control of Fungi Associated With The Post-Harvest Rot of Solenostemon Rotundifolius (Poir) J.K. Morton in Adamawa State of Nigeria.Document6 pagesIdentification and Control of Fungi Associated With The Post-Harvest Rot of Solenostemon Rotundifolius (Poir) J.K. Morton in Adamawa State of Nigeria.Alexander DeckerNo ratings yet
- Morphological Credentials of Afla-Toxigenic and Non-ToxigenicDocument16 pagesMorphological Credentials of Afla-Toxigenic and Non-ToxigenicSugi HartonoNo ratings yet
- 07 Koomnok Diazotroph Endophytic Bacteria in CultivatedDocument7 pages07 Koomnok Diazotroph Endophytic Bacteria in CultivatedAnnisa DewiNo ratings yet
- Comparative Analysis Among Pathogenic Fungal Species That Cause Gladiolus (Gladiolus Grandiflorus Hort.) Corm Rot in MexicoDocument8 pagesComparative Analysis Among Pathogenic Fungal Species That Cause Gladiolus (Gladiolus Grandiflorus Hort.) Corm Rot in MexicoCitlalli BrionesNo ratings yet
- A Technique For Studying The Life Cycle of Meloidogyne Graminicola in Rice RootsDocument3 pagesA Technique For Studying The Life Cycle of Meloidogyne Graminicola in Rice RootsGrace CañasNo ratings yet
- Plant Breeding ReviewsFrom EverandPlant Breeding ReviewsIrwin GoldmanNo ratings yet
- Effect of Heat Treatment On Curcuminoid, Colour Value and Total Polyphenols of Fresh Turmeric RhizomeDocument8 pagesEffect of Heat Treatment On Curcuminoid, Colour Value and Total Polyphenols of Fresh Turmeric RhizomeMuhammad Maulana SidikNo ratings yet
- Statepfofileofvidarbha PDFDocument53 pagesStatepfofileofvidarbha PDFAditiNo ratings yet
- Assignment On Computer HardwareDocument9 pagesAssignment On Computer HardwareMuktadirhasan100% (1)
- Tactile Internet MSC V2Document28 pagesTactile Internet MSC V2hendNo ratings yet
- Coordinated Voltage and Reactive Power Control Strategy With Distributed Generator For Improving The Operational EfficiencyDocument8 pagesCoordinated Voltage and Reactive Power Control Strategy With Distributed Generator For Improving The Operational EfficiencyRaphael NgenyiNo ratings yet
- WDM Pon:: Systems and TechnologiesDocument27 pagesWDM Pon:: Systems and Technologiesducnm1977No ratings yet
- 10 1108 - JPBM 07 2022 4070Document19 pages10 1108 - JPBM 07 2022 4070erikNo ratings yet
- Tots FaqDocument12 pagesTots FaqkbsnNo ratings yet
- Computer PackagesDocument72 pagesComputer PackagesBildad JoashNo ratings yet
- Heat Transfer Through Extended SurfacesDocument16 pagesHeat Transfer Through Extended SurfaceschawarepNo ratings yet
- Donna's Score During The Third Quarter ExaminationDocument7 pagesDonna's Score During The Third Quarter ExaminationGeraldine Valdez CacabilosNo ratings yet
- 4 Tutorial Present Worth AnalysisDocument4 pages4 Tutorial Present Worth AnalysisMuhamad SyazwanNo ratings yet
- Compro Saj 2023 - 22052023Document58 pagesCompro Saj 2023 - 22052023Ahmad FauziNo ratings yet
- Director Contract Management in Florida North Carolina Resume Thomas CzajaDocument3 pagesDirector Contract Management in Florida North Carolina Resume Thomas CzajaThomas CzajaNo ratings yet
- Tuto Traktor Arduino enDocument11 pagesTuto Traktor Arduino enlexetaNo ratings yet
- UntreatedDocument29 pagesUntreatedhahahaNo ratings yet
- Sachin KotianDocument4 pagesSachin Kotianapi-3699646No ratings yet
- Plinth Beams (1st Joint)Document11 pagesPlinth Beams (1st Joint)Tariq MahmoodNo ratings yet
- q4_tleDocument65 pagesq4_tleAngelica TaerNo ratings yet
- Manual Tud300Document70 pagesManual Tud300DionicioCasanovaNo ratings yet
- Mitsubishi: Technical Service BulletinDocument11 pagesMitsubishi: Technical Service BulletinKonstantinNo ratings yet
- DMAE Powder Safety Data SheetDocument3 pagesDMAE Powder Safety Data SheetAInhoaNo ratings yet
- How The World Was Created (Panayan)Document25 pagesHow The World Was Created (Panayan)Mary Kris De AsisNo ratings yet