Professional Documents
Culture Documents
Amalgam
Uploaded by
Harry ArdiyantoCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Amalgam
Uploaded by
Harry ArdiyantoCopyright:
Available Formats
Amalgam Excessive contraction can lead to microleakage and secondary caries Excessive expansioncan produce pressure on the pulp
and post-operative sensitivity, or protrusion of restoration
SILVER Increases Strength Increases Expansion Decreases Flow Decreases Setting time Increases Corrosion resistance COPPER Increases Strength Increases Expansion Decreases Flow Decreases Setting time Increases Corrosion resistance Decreases Plasticity Increases Hardness Increases Brittleness ZINC Increases Strength Increases Expansion Increases Flow Increases Setting time Decreases Corrosion resistance Increases Plasticity Decreases Hardness Decreases Brittleness INDIUM Increases Strength Increases Expansion Increases Flow Increases Setting time Amalgamation more difficult Deoxidiser MERCURY Decreases setting time Decreases delayed expansion GOLD Increases Strength Increases Corrosion resistance TIN Decreases Strength Decreases Expansion Increases Flow Decreases Setting time Decreases Corrosion resistance Increases Plasticity
Setting Reaction
This is a process by which liquid Hg reacts with dental amalgam alloy particles to produce a matrix of intermetallic compounds of Hg with metals of the alloy.
Low Copper alloys
Ag3Sn Excess phase
Hg
Ag2Hg3 1 Phase
Sn7Hg8 2 Phase
Ag3Sn Unreacted Phase
Unreacted phase is bound by a matrix of 1 and 2 phases. Phase: Highest strength (32-35% volume of set amalgam)
1 phase: Moschellandsbegite phase. Highest resistance to corrosion. (5456%) 2 phase: Least strength and resistance to corrosion (11-13%)
Factors affecting strength of Dental Amalgam 1. Particle size: Decreased size results in increased strength (due to increased surface area / unit volume) 2. Particle shape: Regular uniform shape result increased strength (due to more wettability, more coherent mass, less interrupted coherent interphases) 3. Microstructure of amalgam: o Increased and 1 phases there is increased strength o presence of phase there is increased strength (due to prevention of grain boundary sliding) o Increased 2 phase, there is decreased strength 4. Porosities and voids in amalgam: Decreased strength. Formed due to: o Decreased trituration o Decreased condensation pressure o Irregularly shaped particles o Insertion of too large increments o Delayed insertion after trituration o Too less Hg (amalgam non-plastic) o Miscalculation of powder particle diameter to occupy available spaces 5. Hg/Alloy ratio: Increased Hg/Alloy ratio, decreased strength, because increased Hg results in o Decreased unreacted phase o Increased 2 phase o Increased residual Hg (weakest phase) within amalgam 6. Trituration o Increased trituration within limits increases strength (due to increased coherence of matrix crystals). o Increased trituration beyond limits decreases strength ( due to cracking of formed crystals decreasing coherence). 7. Condensation pressure Increased pressure results in increased strength (due to removal of excess Hg within amalgam resulting in less residual Hg) 8. Temperature Amalgam loses 15% of its strength when its temperature is increased from room temperature to mouth temperature. It loses 50% of its strength when temperature is elevated beyond 60C (as in overjealous polishing). 9. Corrosion activity: Decreased corrosion activity results in increased adhesive integrity and therefore increased strength.
Creep
Creep occurs because of grain boundary sliding. crystals on 1 grains prevent grain boundary sliding and therefore are responsible for decreased creep values of high
copper alloys. Higher creep is associated with flow of amalgam over cavity margins which is thin and easily fractures under occlusal stress ("ditched amalgam"). Factors affecting Creep 1. Microstructure of amalgam o Increased 1 fraction, increased creep o Increased 2 fraction, increased creep o Increased grain size of 1, decreased creep o Presence of phase, decreased creep 2. Hg/Alloy ratio Increased Hg/Alloy ratio, increased creep (due to more residual Hg) 3. Trituration o Overtrituration, increased creep o Undertrituration Increased creep Decreased creep in lathe-cut amalgam 4. Condensation pressure Increased pressure, decreased creep (due to less residual Hg) 5. Delay between trituration and condensation Increased creep
Resistance to corrosion
Passive layer of chlorides, sulphides, and /or oxides seen on amalgam surface in unhygienic mouths. Electrolytic corrosion of dissimilar portions of the filling. Corrosion products that form at the margins over a period serve to seal the marginal gaps. Therefore marginal integrity of amalgam restorations improves with time. Corrosion resistance of various phases in descending order are as follows:
phase (maximum resistance to corrosion) 1 phase silver-copper eutectic phase phase phase 2 phase (least resistance to corrosion)
Clinical considerations
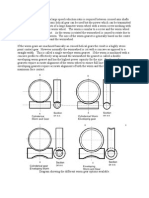
1. Ditched
Amalgam
Fracture of amalgam at the margins Causes are:
Inadequate extension of the cavity walls. Giving cavosurface bevel to to the cavity. High creep value of the amalgam
Larger volume fraction of 1 Presence of 2 phase (as in low Cu alloys) Overtrituration Undertrituration (in spherical alloys) Delay between trituration and condensation High Hg:Alloy ratio Failure to squeeze out exess Hg after trituration Inadequate condensation pressure (excess residual Hg) Delayed expansion due to moisture contamination (flow over the cavity margins) Mercuroscopic expansion (Excess residual Hg Using high Hg:Alloy ratio Failure to squeeze out excess Hg after trituration Inadequate condensation pressure Corrosion of 2 phase Overfilling Excessive (overjealous) burnishing and polishing (flow over the cavity margins) Shallow cavity Thick cement base/cavity liner
2. Marginal leakage: Gap formed between the wall of the cavity and the restored amalgam because of the contraction of the filling material. The gap formed is a potential area for food impaction and plaque accumulation, which in turn result in secondary (recurrent) caries. As the restoration ages, deposition of corrosion products in the gap aid in sealing the margins. 3. Corrosion: If the amalgam surface is not well polished, the rough surface not only attracts plaque but may also undergo crevicular corrosion.
You might also like
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (890)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- ASME B46.1-2009 Surface Texture (Surface Roughness, Waviness, and Lay) - Part2 PDFDocument62 pagesASME B46.1-2009 Surface Texture (Surface Roughness, Waviness, and Lay) - Part2 PDFR JNo ratings yet
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- OU Open University SM358 2012 Exam SolutionsDocument20 pagesOU Open University SM358 2012 Exam Solutionssam smithNo ratings yet
- Advanced Concrete Technology - 10cv81 - ACE NotesDocument185 pagesAdvanced Concrete Technology - 10cv81 - ACE Notessaqeeb33% (3)
- J. B. Tatum - Electricity and Magnetism Vol. 1 PDFDocument186 pagesJ. B. Tatum - Electricity and Magnetism Vol. 1 PDFlucas_vai100% (1)
- A Worm GearDocument19 pagesA Worm GearPetrus Malailak0% (1)
- Experiment 4: Work, Power and Energy Laboratory Report Group 5Document6 pagesExperiment 4: Work, Power and Energy Laboratory Report Group 5Socorro DuqueNo ratings yet
- ORE Exam Part Content 2Document7 pagesORE Exam Part Content 2Ushma Vyas100% (1)
- ORE DMGuidanceDocument19 pagesORE DMGuidanceHarry ArdiyantoNo ratings yet
- ORE DMGuidanceDocument19 pagesORE DMGuidanceHarry ArdiyantoNo ratings yet
- BES - IsO - UT - 12 - Ultrasonic Test - Rev 00Document43 pagesBES - IsO - UT - 12 - Ultrasonic Test - Rev 00Ciety Ma100% (1)
- Patient Information For InvisalignDocument10 pagesPatient Information For InvisalignHarry ArdiyantoNo ratings yet
- CrownandExtracoronalRestorations EndodWhitworthDocument11 pagesCrownandExtracoronalRestorations EndodWhitworthMitza CristianNo ratings yet
- A Comparative Iin Vitro:i Study of Microleakage by A Radioactive Isotope and Compressive Strength of Three Nanofilled Composite Resin RestorationsDocument3 pagesA Comparative Iin Vitro:i Study of Microleakage by A Radioactive Isotope and Compressive Strength of Three Nanofilled Composite Resin RestorationsHarry ArdiyantoNo ratings yet
- Sinusliftandmanipulationpage1 JPG 120526084529 Phpapp01Document1 pageSinusliftandmanipulationpage1 JPG 120526084529 Phpapp01Harry ArdiyantoNo ratings yet
- College of Diplomat Es Manual 061520 Doc 4843Document62 pagesCollege of Diplomat Es Manual 061520 Doc 4843Harry ArdiyantoNo ratings yet
- The EndoVac Method of IrrigationDocument7 pagesThe EndoVac Method of IrrigationHarry ArdiyantoNo ratings yet
- Technical Quality of Root Canal Treatment and Detection of Iatrogenic Errors in An UndergraduateDocument10 pagesTechnical Quality of Root Canal Treatment and Detection of Iatrogenic Errors in An UndergraduateHarry ArdiyantoNo ratings yet
- O2L1KBDocument7 pagesO2L1KBHarry ArdiyantoNo ratings yet
- Vol09 2 75-78strDocument4 pagesVol09 2 75-78strHarry ArdiyantoNo ratings yet
- Esthetic Dentistrydocdoc1257Document25 pagesEsthetic Dentistrydocdoc1257Harry ArdiyantoNo ratings yet
- Esthetic Dentistrydocdoc1257Document25 pagesEsthetic Dentistrydocdoc1257Harry ArdiyantoNo ratings yet
- 4dent 4f172b5c76Document51 pages4dent 4f172b5c76Harry ArdiyantoNo ratings yet
- Regenerative Endodontic Procedures for TeethDocument1 pageRegenerative Endodontic Procedures for TeethHarry ArdiyantoNo ratings yet
- Re SurgeryDocument7 pagesRe SurgeryHarry ArdiyantoNo ratings yet
- OptraSculpt PadDocument2 pagesOptraSculpt PadHarry ArdiyantoNo ratings yet
- ORE Equipment and Materials ForDMDocument3 pagesORE Equipment and Materials ForDMHarry ArdiyantoNo ratings yet
- 4dent 4f172b5c76Document51 pages4dent 4f172b5c76Harry ArdiyantoNo ratings yet
- FAQs For Funding 1213Document14 pagesFAQs For Funding 1213Harry ArdiyantoNo ratings yet
- Funding & Fees at King's College LondonDocument2 pagesFunding & Fees at King's College LondonHarry ArdiyantoNo ratings yet
- ORE Part2contentDocument1 pageORE Part2contentHarry ArdiyantoNo ratings yet
- Overseas Registration Examination: ORE Part 1 - FormatDocument4 pagesOverseas Registration Examination: ORE Part 1 - Formatnanania01No ratings yet
- Sickness GuidanceDocument2 pagesSickness GuidanceHarry ArdiyantoNo ratings yet
- ORE MEGuidance181209webrevisedDocument5 pagesORE MEGuidance181209webrevisedHarry ArdiyantoNo ratings yet
- LDS Candidate Guidance GeneralDocument2 pagesLDS Candidate Guidance GeneralHarry ArdiyantoNo ratings yet
- OSCEguidanceDocument5 pagesOSCEguidanceAshraf MorsyNo ratings yet
- Part A Content 1Document2 pagesPart A Content 1devassykuttyNo ratings yet
- M.implant Munster & GboiDocument7 pagesM.implant Munster & GboiHarry ArdiyantoNo ratings yet
- 0 Physics SyllabusDocument2 pages0 Physics Syllabusiffat fatima patilNo ratings yet
- (Cis Cu (Gly) 2) H2ODocument13 pages(Cis Cu (Gly) 2) H2OMichaelNo ratings yet
- Emr/Esr/Epr Spectroscopy For Characterization of NanomaterialsDocument183 pagesEmr/Esr/Epr Spectroscopy For Characterization of NanomaterialsRajendraNo ratings yet
- GoldenGate Phase Noise Ext v2Document25 pagesGoldenGate Phase Noise Ext v2RONALEDNo ratings yet
- Generator synchronization challengesDocument6 pagesGenerator synchronization challengesabdulyunus_amirNo ratings yet
- Chapter 4 Fourier Optics: D D y X J F y X F FDocument14 pagesChapter 4 Fourier Optics: D D y X J F y X F FansarixxxNo ratings yet
- Turbidity Currents and Hydraulic JumpsDocument37 pagesTurbidity Currents and Hydraulic JumpsVenkataraju BadanapuriNo ratings yet
- Casting RefDocument20 pagesCasting RefNavdeep GillNo ratings yet
- Curve FittingDocument28 pagesCurve FittingCillalois Marie FameroNo ratings yet
- The LengthDocument1 pageThe LengthRAIZZ67% (3)
- Notebook 11Document2 pagesNotebook 11api-334252501No ratings yet
- Manzil JEE (2024) Electric Charges and Field: JEE Revision Practice SheetDocument8 pagesManzil JEE (2024) Electric Charges and Field: JEE Revision Practice SheetMr. ThomasNo ratings yet
- Principles of AVO Crossplotting PDFDocument6 pagesPrinciples of AVO Crossplotting PDFNurlia AduNo ratings yet
- Chapter 1 - Introduction To PolymersDocument94 pagesChapter 1 - Introduction To PolymersArnav Hasija0% (1)
- Elastic Constt of Epoxy ResinDocument4 pagesElastic Constt of Epoxy ResinsrbsubbuNo ratings yet
- Abrasion Investigation Article-DataDocument143 pagesAbrasion Investigation Article-DataAttyubNo ratings yet
- Turbomacchine PDFDocument2 pagesTurbomacchine PDFCarlos RodriguesNo ratings yet
- Batch Distillation Principles and Example Calculation"TITLE"Simplified Rayleigh Equation for Batch Distillation" TITLE"Multicomponent Batch Distillation Calculation Using Relative VolatilityDocument9 pagesBatch Distillation Principles and Example Calculation"TITLE"Simplified Rayleigh Equation for Batch Distillation" TITLE"Multicomponent Batch Distillation Calculation Using Relative VolatilityFaturrohman SetyoajiNo ratings yet
- MH1811 Final Exam 16-17 Sem 1Document12 pagesMH1811 Final Exam 16-17 Sem 1Mia SohNo ratings yet
- Notes On Semiconductor Physics For Electronic DevicesDocument27 pagesNotes On Semiconductor Physics For Electronic DevicesspyseetunaNo ratings yet
- General Chemistry Quarter 2 Week 1 3Document7 pagesGeneral Chemistry Quarter 2 Week 1 3Istian VlogsNo ratings yet
- Solution of Engineering MatgematicsDocument119 pagesSolution of Engineering MatgematicsEng W Ea100% (2)