Professional Documents
Culture Documents
MLE1101 Tutorial 2 - Suggested Solutions for BCC Crystal Structure, Lattice Constant and Element Identification
Uploaded by
Yin HauOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
MLE1101 Tutorial 2 - Suggested Solutions for BCC Crystal Structure, Lattice Constant and Element Identification
Uploaded by
Yin HauCopyright:
Available Formats
1
MLE1101 Tutorial 2 - Suggested Solutions
1. Draw direction vectors in unit cubes for the following cubic directions:
(a) ] 1 1 1 [ (b) ] 0 1 1 [ (c) ] 1 2 1 [ (d) ] 3 1 1 [
Solution:
(a) ] 1 1 1 [
(b) ] 0 1 1 [
(c) ] 1 2 1 [
(d) ] 3 1 1 [
2
2. What are the indices of the directions shown in the unit cube below:
Solution:
Direction (e):
Subtracting origin coordinates from emergence coordinates,
( ) ( ) ( )
=
3 1
4 4
,0,1 1,1,0 , 1,1
Direction indices: [344]
Direction (f):
Subtracting origin coordinates from emergence coordinates,
( ) ( ) ( )
=
1 2 1
3 3 3
0,1, 1,0, 1,1,
Direction indices: [331]
Direction (g):
Subtracting origin coordinates from emergence coordinates,
( ) ( ) ( )
=
1 1 1
4 2 4
1, 0, 0,1, 1, 1,
Direction indices: [441]
Direction (h):
Subtracting origin coordinates from emergence coordinates,
( ) ( ) ( )
=
3 3 3 3
4 4 4 4
,0,0 0,1, , 1,
Direction indices: [343]
3. What are the Miller indices of the cubic crystallographic planes shown below:
3
Solution:
Plane a with (1,0,1) as origin Plane b with (0,1,1) as origin
Planar intercepts Reciprocal of intercepts Planar intercepts Reciprocal of intercepts
x = 1
1
1
x
= x = 1
1
1
x
=
y =
1
0
y
= y = 1
1
1
y
=
z =
1
3
1
3
z
= z =
2
3
3 1
2 z
=
The miller indices of plane a are (103) The miller indices of plane b are (223)
Plane c with (0,1,0) as origin Plane d with (0,1,0) as origin
Planar intercepts Reciprocal of intercepts Planar intercepts Reciprocal of intercepts
x =
9
5
* =
5 1
9 x
x = 1
1
1
x
=
y =
3
4
=
1 4
3 y
y = 1
1
1
y
=
z =
1
0
z
= z =
1
2
1
2
z
=
The miller indices of plane c are (5120) The miller indices of plane d are (112)
*For plane c, to obtain x =
9
5
, refer to diagram below:
4. Draw in unit cubes the crystal planes that have the following Miller indices:
Extending plane c to
meet the new x-axis
(red line).
4
(a) ) 1 1 1 ( (b) ) 2 10 ( (c) ) 1 2 1 ( (d) ) 3 21 (
Solution:
(a) For (111) , planar intercepts are: 1, 1, 1 x y z = = =
(b) For (102) , planar intercepts are:
1
2
1, , x y z = = =
(c) For (121) , planar intercepts are:
1
2
1, , 1 x y z = = =
(d) For (213) , planar intercepts are:
1 1
2 3
, 1, x y z = = =
5. The d
310
interplanar spacing in a BCC element is 0.1587 nm.
5
(a) What is its lattice constant a?
(b) What is the atomic radius of the element?
(c) What could this element be?
Solution:
(a) ( )
2 2 2 2 2 2
310
0.1587nm 3 1 0 0.502nm a d h k l = + + = + + =
(b) For BCC structure,
( ) 3 0.502nm 3
0.217nm
4 4
a
R = = =
(c) The element is barium.
6. Draw the hexagonal crystal planes whose Miller-Bravais indices are:
(a) ) 1 1 10 ( (b) ) 1 1 01 ( (c) ) 0 1 2 1 (
Solution:
The reciprocals of the indices provided give the intercepts for the plane (a
1
, a
2
, a
3
, c)
(a)
1 2 3
1, , 1, 1 a a a c = = = = (b)
1 2 3
, 1, 1, 1 a a a c = = = =
(c)
1
1 2 3 2
1, , 1, a a a c = = = =
6
7. Determine the Miller-Bravais indices of the hexagonal crystal planes in the figure below:
Solution:
Plane a with (0,0,0,0) as origin Plane b with (0,0,0,1) as origin Plane c with (0,0,0,0) as origin
Planar
intercepts
Reciprocal
intercepts
Planar
intercepts
Reciprocal
intercepts
Planar
intercepts
Reciprocal
intercepts
1
a =
1
1
0
a
=
1
1 a =
1
1
1
a
=
1
1 a =
1
1
1
a
=
2
1 a =
1
2
1
a
=
2
1 a =
1
2
1
a
=
2
1 a =
1
2
1
a
=
3
1 a =
1
3
1
a
=
3
a =
1
3
0
a
=
3
a =
1
3
0
a
=
c =
1
0
c
= 1 c =
1
1
c
= 1 c =
1
1
c
=
The miller indices of plane a
are (0110)
The miller indices of plane b
are (1101)
The miller indices of plane c
are (1101)
8. The lattice constant for BCC tantalum at 20C is 0.33026 nm and its density is 16.6 g/cm
3
.
Calculate a value for its atomic mass.
Solution:
The atomic mass can be found based on the mass of tantalum in a unit BCC cell.
( )
( )( )( )
3
3
3 6 3 3 9
22
mass per unit cell vol per unit cell
16.6g /cm 10 cm /m 0.33026 10 m
5.98 10 g/unit cell
v v
a
= =
=
=
Since there are two atoms in a BCC unit cell, the atomic mass is
( )( )
22 23
5.98 10 g/unit cell 6.023 10 atoms/mol
atomic mass 180.09g/mol
2atoms/unit cell
= =
7
9. Calculate the planar atomic density in atoms per square millimetre for the (111) crystal plane
in FCC gold, which has a lattice constant of 0.40788 nm.
Solution:
The triangular (111) plane contains:
1 1
2 6
(3 face atoms atom) (3 corners atom per corner) 2 atoms + =
( )
2
1 1 3 3
area 2
2 2 2 2
bh a a a
| |
= = =
|
\ .
( )
( )( )
19 2 6 2 2
2
9
13 2
2 atoms
density 1.388 10 atoms /m 10 m /mm
3
0.40788 10 m
2
1.39 10 atoms /mm
= =
=
10. Calculate the linear atomic density in atoms per millimetre for the [110] direction in BCC
vanadium, which has a lattice constant of 0.3039 nm.
Solution:
linear atomic density,
no. of atomic diameter intersected by selected length of direction line
selected length of line
l
=
For [110] direction of BCC Va,
( )
( )
6
6
1 atom 1 atom
2.33 10 atoms/mm
2 2 0.3039nm 10 mm/nm
l
a
= = =
8
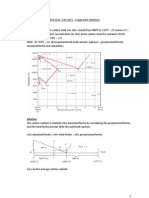
11. An X-ray diffractometer recorder chart for an element that has either the BCC or the FCC
crystal structure showed diffraction peaks at the following 2 angles:
38.60, 55.71, 69.70, 82.55, 95.00 and 107.67. (Wavelength of the incoming radiation
was 0.15405 nm.)
(a) Determine the crystal structure of the element.
(b) Determine the lattice constant of the element.
(c) Identify the element.
Solution:
(a) Comparing the sin
2
term for the first two angles:
2 sin sin
2
38.60 19.30 0.33051 0.10924
55.71 27.855 0.46724 0.21831
2
1
2
2
sin 0.10924
0.50 BCC
0.21831 sin
0
0
= =
(b) The lattice constant depends on the first sin
2
term as well as the Miller indices of
the first set of BCC principal diffracting planes {110}
2 2 2 2 2 2
2
1
0.15405nm 1 1 0
0.3296nm
2 2 0.10924 sin
h k l
a
0
+ + + +
= = =
(c) The element is niobium (Nb). It has a lattice constant of 0.33007 nm; amongst the
BCC metals, it has the value closest to that obtained in (b).
You might also like
- Chapt 03 Sect 7 To 11Document15 pagesChapt 03 Sect 7 To 11Jesse McClure100% (1)
- 05 CEE2219 TM2 MidExam - 2018-19 - SolnDocument8 pages05 CEE2219 TM2 MidExam - 2018-19 - SolnCyrus ChartehNo ratings yet
- 3.30 Within A Cubic Unit Cell, Sketch The Following DirectionsDocument13 pages3.30 Within A Cubic Unit Cell, Sketch The Following DirectionsTrong LanNo ratings yet
- Review Problems For Chapter3Document11 pagesReview Problems For Chapter3johandreher100% (1)
- CH 9 Solutions - CalculusDocument37 pagesCH 9 Solutions - CalculusAnaga RajanNo ratings yet
- Assignment 1 With SolutionDocument8 pagesAssignment 1 With SolutionFaryal BatoolNo ratings yet
- Chapter 3 Homeworks: Compute densities and interplanar spacings for iron and aluminumDocument7 pagesChapter 3 Homeworks: Compute densities and interplanar spacings for iron and aluminumFadzil AtiqahNo ratings yet
- Materials Science HW ProblemsDocument8 pagesMaterials Science HW Problemsamitav_chak9523No ratings yet
- ECE606 f12 hw1 SolDocument8 pagesECE606 f12 hw1 SolanshNo ratings yet
- AE 321 - Solution of Homework #5: (5×5 25 POINTS)Document9 pagesAE 321 - Solution of Homework #5: (5×5 25 POINTS)Arthur DingNo ratings yet
- Cive162001 2006 Brief SolutionsDocument7 pagesCive162001 2006 Brief Solutionssjeyarajah21No ratings yet
- Homework 5Document2 pagesHomework 5Arthur DingNo ratings yet
- N M M N M M B A B A N: Solution Marks RemarksDocument6 pagesN M M N M M B A B A N: Solution Marks Remarks葉海洋No ratings yet
- Structure ProblemDocument4 pagesStructure Problemjishnu098No ratings yet
- Vector Analysis Problem Set SolutionsDocument5 pagesVector Analysis Problem Set SolutionsTy PhanNo ratings yet
- WBJEE 2010 Question Paper With SolutionDocument50 pagesWBJEE 2010 Question Paper With SolutionLokesh Kumar67% (3)
- 1 DEC-2012 Physical Sciences Booklet Series (A) Solved Part-ADocument35 pages1 DEC-2012 Physical Sciences Booklet Series (A) Solved Part-ASaurav PatelNo ratings yet
- Index Miller XRDDocument22 pagesIndex Miller XRDAchsan ArfandiNo ratings yet
- Midterm P2800 2008 SolutionsDocument6 pagesMidterm P2800 2008 Solutionskhalil alhatabNo ratings yet
- BSTATsample 09Document20 pagesBSTATsample 09Akhilesh ReddyNo ratings yet
- MEC4418 - Assignment 1 - SolutionDocument6 pagesMEC4418 - Assignment 1 - Solutionsamuel_parsooramenNo ratings yet
- 반도체 솔루션 4판 PDFDocument121 pages반도체 솔루션 4판 PDFkokori8880% (5)
- Qs and Ans On An Introduction To Material ScienceDocument6 pagesQs and Ans On An Introduction To Material Sciencen.learner.101No ratings yet
- Differential Equations and Fourier Series Exam QuestionsDocument24 pagesDifferential Equations and Fourier Series Exam QuestionsPrudhvi BadeNo ratings yet
- CSG ch10Document125 pagesCSG ch10Joanna PoshnjaNo ratings yet
- C4 Bronze 4Document18 pagesC4 Bronze 4Betty MakharinskyNo ratings yet
- Acs I Y4 Prelim Em1 2010Document15 pagesAcs I Y4 Prelim Em1 2010math3matics3No ratings yet
- Problems On STMDocument4 pagesProblems On STMshanthakumargc0% (1)
- Homework 1 SolutionsDocument20 pagesHomework 1 Solutionsjohn0% (1)
- STR AnalysisDocument136 pagesSTR AnalysisWeha Yu100% (1)
- CBSE-2023 PRACTICE PAPER 3 MATHEMATICS - 10 SEO-Optimized TitleDocument10 pagesCBSE-2023 PRACTICE PAPER 3 MATHEMATICS - 10 SEO-Optimized TitlePratik BorleNo ratings yet
- Notes and Formulae SPM Mathematics Form 1 - 3 Notes Solid GeometryDocument9 pagesNotes and Formulae SPM Mathematics Form 1 - 3 Notes Solid GeometryJarnice Ling Yee ChingNo ratings yet
- SPM Notes & Formulae MathematicsDocument9 pagesSPM Notes & Formulae MathematicsPatrick PhuahNo ratings yet
- Solutions All MOCK Tests - MEDocument168 pagesSolutions All MOCK Tests - MEsrishashank100% (1)
- Revision - Term 1 - Grade 10 (Tests)Document7 pagesRevision - Term 1 - Grade 10 (Tests)Cool TreesNo ratings yet
- AP Calc BC Sem 1 Final ReviewDocument12 pagesAP Calc BC Sem 1 Final ReviewParth PatelNo ratings yet
- Matrices Determinants MSDocument42 pagesMatrices Determinants MSRenario Gule Hinampas Jr.No ratings yet
- List of FormulaDocument2 pagesList of FormulaAin NajlaNo ratings yet
- 2009 CT 2 ANS - StudentDocument4 pages2009 CT 2 ANS - StudentJoanne ErNo ratings yet
- X NSTSE Level-1 Mock Test-2-5Document15 pagesX NSTSE Level-1 Mock Test-2-5puneet manglaNo ratings yet
- IntoMath Grade 10 Practice 2Document13 pagesIntoMath Grade 10 Practice 2Jay el CuadraNo ratings yet
- AP EAMCET 2016 Engineering Test Solutions by Sri Chaitanya PDFDocument52 pagesAP EAMCET 2016 Engineering Test Solutions by Sri Chaitanya PDFSree Venkat100% (1)
- CH 08Document97 pagesCH 08albakili775514617No ratings yet
- 2011 10 Lyp Mathematics Sa1 13Document9 pages2011 10 Lyp Mathematics Sa1 13Rohit MaheshwariNo ratings yet
- KEE - Model Question PaperDocument19 pagesKEE - Model Question PaperPremKumarKalikiriNo ratings yet
- Solutions To Home Practice Test/Mathematics: Circles HWT - 1Document6 pagesSolutions To Home Practice Test/Mathematics: Circles HWT - 1varunkohliinNo ratings yet
- Math Sa1 10thDocument10 pagesMath Sa1 10thpranav1999No ratings yet
- Sa 15Document10 pagesSa 15vijaynaraNo ratings yet
- 2011 Mock Paper BythemathscafeDocument6 pages2011 Mock Paper BythemathscafefardeenNo ratings yet
- B.Mat Part Test 3: IIT 2011 PT3/CMP/P (I) /SOLNSDocument32 pagesB.Mat Part Test 3: IIT 2011 PT3/CMP/P (I) /SOLNSSarvesh DubeyNo ratings yet
- QP (041) Set 2 X MathsDocument5 pagesQP (041) Set 2 X MathsbitchingaroundNo ratings yet
- Practise QpsDocument104 pagesPractise QpsFroFee FNo ratings yet
- Mathematics Olympiad Set 2Document21 pagesMathematics Olympiad Set 2ichkhuyNo ratings yet
- All India Ijso (Stage-I) Test SeriesDocument21 pagesAll India Ijso (Stage-I) Test Seriesthorgod94150No ratings yet
- Some Important Formulae, Definitions and Results in MathematicsDocument12 pagesSome Important Formulae, Definitions and Results in Mathematicsparchure123No ratings yet
- Aakash Solutions For JEE ADVANCED PAPER 1Document30 pagesAakash Solutions For JEE ADVANCED PAPER 1Tushar KumarNo ratings yet
- Geometry Prelim Exam Review with SolutionsDocument4 pagesGeometry Prelim Exam Review with SolutionsSAID SAYALINo ratings yet
- 12a Olym 2Document299 pages12a Olym 2micaelcarinhanhaNo ratings yet
- Electrochemical Processes in Biological SystemsFrom EverandElectrochemical Processes in Biological SystemsAndrzej LewenstamNo ratings yet
- Electron Beam-Specimen Interactions and Simulation Methods in MicroscopyFrom EverandElectron Beam-Specimen Interactions and Simulation Methods in MicroscopyNo ratings yet
- Thermodynamics and Its Application in RefrigerationDocument8 pagesThermodynamics and Its Application in RefrigerationYin HauNo ratings yet
- Heat Transfer Radiation Problem SetDocument1 pageHeat Transfer Radiation Problem SetLakshmi BalasubramaniamNo ratings yet
- Gek 1540 Tut 2 QBDocument2 pagesGek 1540 Tut 2 QBYin HauNo ratings yet
- Laplace Transform TableDocument3 pagesLaplace Transform TableYin HauNo ratings yet
- ME3112-PART 2 Tutorial 2 & 3: ShahrokhDocument14 pagesME3112-PART 2 Tutorial 2 & 3: ShahrokhYin HauNo ratings yet
- Heat Transfer Radiation Problem SetDocument1 pageHeat Transfer Radiation Problem SetLakshmi BalasubramaniamNo ratings yet
- Gek1540 Chapter 6Document4 pagesGek1540 Chapter 6Yin HauNo ratings yet
- Entropy 15 01221Document11 pagesEntropy 15 01221Yin HauNo ratings yet
- Engineers & EnvironmentDocument8 pagesEngineers & EnvironmentYin HauNo ratings yet
- Tutorial 7 Suggested AnswersDocument4 pagesTutorial 7 Suggested AnswersYin HauNo ratings yet
- Mechanical Properties and Testing of Materials Chapter 2Document7 pagesMechanical Properties and Testing of Materials Chapter 2Yin HauNo ratings yet
- ME3162 Questions PDFDocument2 pagesME3162 Questions PDFYin HauNo ratings yet
- ME2142E Feedback Control Systems-CheatsheetDocument2 pagesME2142E Feedback Control Systems-CheatsheetPhyo Wai Aung67% (9)
- CA Lab Manual ScopeDocument10 pagesCA Lab Manual Scopea2367916100% (1)
- LSM1301Document7 pagesLSM1301Yin HauNo ratings yet
- Tutorial 7 Suggested AnswersDocument4 pagesTutorial 7 Suggested AnswersYin HauNo ratings yet
- MLE1101 - Tutorial 5 - Suggested SolutionsDocument5 pagesMLE1101 - Tutorial 5 - Suggested SolutionsYin HauNo ratings yet
- Chapter 15Document2 pagesChapter 15Yin HauNo ratings yet
- MLE1101 - Tutorial 6 - Suggested SolutionsDocument5 pagesMLE1101 - Tutorial 6 - Suggested SolutionsYin HauNo ratings yet
- MLE1101 Tutorial 4 - Suggested Solutions AnalysisDocument7 pagesMLE1101 Tutorial 4 - Suggested Solutions AnalysisYin HauNo ratings yet
- TimetableDocument2 pagesTimetableYin HauNo ratings yet
- MNO1001X Cheat SheetDocument8 pagesMNO1001X Cheat SheetYin HauNo ratings yet
- MLE1101 - Tutorial 3 - Suggested SolutionsDocument4 pagesMLE1101 - Tutorial 3 - Suggested SolutionsYin HauNo ratings yet
- MLE1101 - Tutorial 1 - Suggested SolutionsDocument5 pagesMLE1101 - Tutorial 1 - Suggested SolutionsYin HauNo ratings yet
- LSM1301Document7 pagesLSM1301Yin HauNo ratings yet
- 12 TransientDocument39 pages12 TransientYin HauNo ratings yet
- L2 - LeadershipDocument2 pagesL2 - LeadershipYin HauNo ratings yet
- EC1301 - Tutorial 4 (14-18 September 2009) - AnswersDocument9 pagesEC1301 - Tutorial 4 (14-18 September 2009) - AnswersYin HauNo ratings yet
- EC1301 Mid-Term Exam Questions (09102009 - Make-Up Exam)Document11 pagesEC1301 Mid-Term Exam Questions (09102009 - Make-Up Exam)Yin Hau100% (1)
- Casting defects and solidificationDocument5 pagesCasting defects and solidificationAhmad AbdNo ratings yet
- 1984givord - SSC - Magnetic Properties and Crystal Structure of Nd2Fe14BDocument3 pages1984givord - SSC - Magnetic Properties and Crystal Structure of Nd2Fe14BPatrícia CanazartNo ratings yet
- Class 12 Set - B: Chemistry - CH 402Document3 pagesClass 12 Set - B: Chemistry - CH 402SonuSharmaNo ratings yet
- Tectosilicates - Framework SilicatesDocument19 pagesTectosilicates - Framework SilicatesAMPamasengiNo ratings yet
- Physical and Chemical PropertiesDocument35 pagesPhysical and Chemical PropertiesBien DielNo ratings yet
- EBSD Explained WebDocument13 pagesEBSD Explained WebRonaldo JuniorNo ratings yet
- 1.3 Crystal Faces and Miller IndicesDocument20 pages1.3 Crystal Faces and Miller IndicesTonatiuh ZamaNo ratings yet
- Crystallography With Paper ModelDocument11 pagesCrystallography With Paper ModelDzakiyahNo ratings yet
- Solid State Physics I - PPT (Repaired)Document159 pagesSolid State Physics I - PPT (Repaired)davididosa40No ratings yet
- Shale: Devanarayanan EM I.Msc Applied GeologyDocument20 pagesShale: Devanarayanan EM I.Msc Applied GeologyDq DevanNo ratings yet
- Synthesis of Zeolite-A Using Kaolin Samples From Darazo, Bauchi State and Ajebo, Ogun State in NigeriaDocument9 pagesSynthesis of Zeolite-A Using Kaolin Samples From Darazo, Bauchi State and Ajebo, Ogun State in Nigeriagebreslassie gereziherNo ratings yet
- 10 5923 J Materials 20130304 04Document7 pages10 5923 J Materials 20130304 04Angga SaputraNo ratings yet
- Yrk Mohan 2ND Puc Chemistry 2023 Model QuestionsDocument5 pagesYrk Mohan 2ND Puc Chemistry 2023 Model QuestionsNaga Raj S100% (1)
- Buy Pukhraj Gemstone Online - Original Pukhraj Stone PriceDocument5 pagesBuy Pukhraj Gemstone Online - Original Pukhraj Stone PriceShakti narayananNo ratings yet
- Cuachon Cegeol220 Activityno02Document6 pagesCuachon Cegeol220 Activityno02Emriel John L. CuachonNo ratings yet
- Rocks and Minerals For Grade 11Document2 pagesRocks and Minerals For Grade 11len100% (1)
- 3 1 MineralsDocument79 pages3 1 MineralsBenjamin CardwellNo ratings yet
- Rocks and Minerals WorksheetDocument4 pagesRocks and Minerals WorksheetPwiladNo ratings yet
- HORNNBLENDEDocument2 pagesHORNNBLENDESORIANO, JEZEREEL JOYCE A.No ratings yet
- Cuprian Elbaite From The: Batalha Mine, Paraíba, BrazilDocument11 pagesCuprian Elbaite From The: Batalha Mine, Paraíba, Brazilnaayram7491No ratings yet
- Chapter 2 - Wave Diffraction - Part 2Document47 pagesChapter 2 - Wave Diffraction - Part 2Goh boon tongNo ratings yet
- Production of Gold and Silver in Peru - Past, Present and FutureDocument42 pagesProduction of Gold and Silver in Peru - Past, Present and Futuresthefany teodoro castañedaNo ratings yet
- Crystallography (Solid State Physics)Document21 pagesCrystallography (Solid State Physics)Derick Marlo CajucomNo ratings yet
- Gem & Mineral ShowDocument5 pagesGem & Mineral ShowreacharunkNo ratings yet
- CALCITE AND GARNET PROPERTIES by Nelson ArradazaDocument8 pagesCALCITE AND GARNET PROPERTIES by Nelson ArradazaNelson L. ArradazaNo ratings yet
- Small Scale Mining Techniques PresentationDocument10 pagesSmall Scale Mining Techniques PresentationAlex NgezeNo ratings yet
- Silver Vein Deposits Kissin & MangoDocument8 pagesSilver Vein Deposits Kissin & MangoEdwin Naranjo100% (1)
- Citrine Vs Yellow TopazDocument3 pagesCitrine Vs Yellow TopazAlok JagawatNo ratings yet
- Daniela FockeDocument10 pagesDaniela FockeAnonymous cvXgWgdNNo ratings yet
- 1 IntoductionDocument2 pages1 IntoductionAshutosh SinghNo ratings yet