Professional Documents
Culture Documents
Ktt211 18 Electron Rules PDF
Uploaded by
Ahmad MuslihinOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Ktt211 18 Electron Rules PDF
Uploaded by
Ahmad MuslihinCopyright:
Available Formats
04-Ktt212-18elect-09.
pptx
5-Sep-10
The 18 electron rule
For organic chemists its the octet rule, for organometallic chemists its the 18 electron rule. Useful for purposes of predicting reactivity. Each derives from a simple count of the number of electrons that may be accommodated by the available valence orbitals (one s and three p for organic chemists; organometallic chemists get five bonus dorbitals in which to place their electrons).
Slide 1
The 18 electron rule
states will follow the 18electron rule; metals in earlier rows and endrows do not. high field ligands will also observe the 18 electron rule.(later in the course) stability of complex.
Organometallic complexes with oxidation Complexes with backbonding electron or
18electron rule can assist in predicting the
Slide 2
p1
04-Ktt212-18elect-09.pptx
5-Sep-10
What is the 18 Electron Rule?
n + 2(CN) = 18
n = the number of valence d electrons on the metal CN is the coordination number
this 'rule' arise from the closed shell configuration d10s2p6 (Noble gas rule) analogous to octet rule for main group applies to most organometallic compounds
Slide 3
Cr(CO)6
Cr (0) a d6 central metal 6 CO with 2 e per CO
6 12
18
Cr(CO)7 [Cr(CO)6]
Cr (0) a d6 metal 7 CO with 2 e per CO Cr (0) a d6 metal 6 CO with 2 e per CO cas neg.
6 14 6 12 1
20
19
Slide 4
p2
04-Ktt212-18elect-09.pptx
5-Sep-10
CH3Re(CO)5 Re(I) CH3 donates 2 elect. 5 carbonyls (CO) Cp2Ru Ru(II) = 6 2 Cp with 6 elect. (2*(5 + 1)) = 12 Fe(0) Fe2(CO)9 3 terminal CO gives 2 elec. 3 bridging CO gives 1 elec. FeFe bond gives 1 elec. Cr(0) Cr(CO)6 6 Co gives 2 elec. each
6 2 10 6 12 8 6 3 1 6 12
18
18
18
18
Slide 5
Ru(II) = 6 2 Cp with 6 elect. (2*(5 + 1)) = 12
Fe OS = 0; No. of electrons = 8 3 bridging COs; 3*1 = 3
3 terminal COs; 3*2 = 6
Slide 6
p3
04-Ktt212-18elect-09.pptx
5-Sep-10
We can predict that less stable complexes are due to number of electrons; complexes can be stabilized by adding or removing electrons. Complexes that do not obey the rule will undergo reactions to provide a situation whereby the rule is observed: 1. V(CO)6 17elec. will oxidize [V(CO)6] which is an 18electron system. 2.[Fe(C5H5)2]+ with 17elec. will be reduced Fe(C5H5)2 which obeys the 18electron
Slide 7
* No of electrons 1st row
3 Sc Y (La)
4 Ti Zr Hf
5 V Nb Ta
6 Cr Mo W
7 Mn Tc Re
8 Fe Ru Os
9 Co Rh Ir
10 Ni Pd Pt
11 Cu Ag Au
12 Zn Cd Hg
2nd row
3rd row
Oxidation State
No of electrons on the metal ions
O I II III IV
3 2 1 0
4 3 2 1 0
5 4 3 2 1
6 5 4 3 2
7 6 5 4 3
8 7 6 5 4
9 8 7 6 5
10 9 8 7 6
11 10 9 8 7
12 11 10 9 8
Slide 8
p4
04-Ktt212-18elect-09.pptx
5-Sep-10
Knowing how many valence electrons "belong to" a transition metal complex allows us to make predictions about the mechanisms of reactions and the possible modes of reactivity.
There are two distinct methods that are used to count electrons, (i) the neutral or covalent method and (ii) the effective atomic number (EAN) or ionic method. These are simply two different accounting systems that give us the same final answer.
Slide 9
Slide 10
p5
04-Ktt212-18elect-09.pptx
5-Sep-10
Method 1: The ionic (charged) model
The basic premise of this method is that (i) we remove all of the ligands from the metal and (ii) add the proper number of electrons to each ligand to bring it to a closed valence shell state. 1. E.g. we remove ammonia(NH3) from metal complex, NH3 has a completed octet and acts as a neutral molecule. 2. When it bonds to the metal center it does so through its lone pair (Lewis acidbase adduct). 3. We call ammonia a neutral twoelectron donor.
Slide 11
Condt Method 1: The ionic (charged) model
In contrast, if we remove a methyl group from the metal and complete its octet, then we formally have CH3. If we bond this methyl anion to the metal, the lone pair forms our metalcarbon bond and the methyl group acts as a twoelectron donor ligand. Notice that to keep charge neutrality we must oxidize the metal by one electron (i.e. assign a positive charge to the metal). This will reduce the delectron count of the metal center by one.
Slide 12
p6
04-Ktt212-18elect-09.pptx
5-Sep-10
Condt Method 2: The covalent (neutral) model
In this method, we remove all of the ligands from the metal, & we do whatever is necessary to make them neutral, e.g. ammonia, NH3
When removed from the metal, it is a neutral molecule with one lone pair of electrons.
NH3 is a neutral two electron donor as with the ionic model.
Slide 13
We diverge from the ionic model when we consider a ligand such as methyl.
When we remove it from the metal and make the methyl fragment neutral, we have a neutral methyl radical. Both the metal and the methyl radical must donate one electron each to form our metalligand bond. Therefore, the methyl group is a one electron donor, not a two electron donor as it is under the ionic formalism. Where did the other electron "go"? It remains on the metal and is counted there.
In the covalent method, metals retain their full complement of d electrons because we never change the oxidation state from zero; i.e. Fe will always count for 8 electrons
regardless of the oxidation state and Ti will always count for four.
Slide 14
p7
04-Ktt212-18elect-09.pptx
5-Sep-10
Notice that this method does not give us any immediate information about the formal oxidation state of the metal, so we must go back and assign that in a separate step. For this reason, many chemists (particularly those that work with high oxidation state complexes) prefer the ionic method.
Slide 15
(a) 10 e (Pt) + 4 e (4 Cl) + 2 e (charge 2) = 16 e (b) 8 e (Pt2+) + 8 e (4 Cl) = 16 e
[PtCl4]2:
[Mn(CO)5]:
(a) 7 e (Mn) + 10 e (5CO) + 1 e(charge 1) = 18 e (b) 8 e (Mn) + 10 e (5 CO) = 18 e
Slide 16
p8
04-Ktt212-18elect-09.pptx
5-Sep-10
[Mn2(CO)10] : 14 e (2 Mn) + 20 e (10 CO) + 2 e (Mn Mn) = 36 e (i.e., 18 e per Mn)
[Co2(CO)8]: 18 e (2 Co) + 16 e (8 CO) + 2 e (CoCo) = 36 e (i.e., 18 e per Co)
OC CO OC CO
OC
OC
Mn
Mn
CO
O C Co C O Co
CO
OC CO OC CO
OC
CO CO
Slide 17
OC
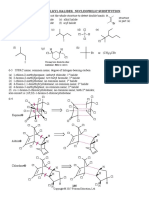
Electron counting for [Rh2Cl2(CO)4]:
(a) 18 e (2 Rh) + 8 e (4 CO) + 6 e (2 bridging Cl) = 32 e (i.e., 16 e per Rh) (b) 16 e (2 Rh+) + 8 e (4 CO) + 8 e (2 bridgingCl) = 32 e (i.e., 16 e per Rh)
Slide 18
p9
04-Ktt212-18elect-09.pptx
5-Sep-10
(C5H5)Fe(CO)2Cl Fe(II) 6 e (C5H5) 6 e 2(CO) 2 x 2 = 4 e Cl 2 e Total 18 e : Electron Counting: Donor Pairs
Fe Cl
CO CO
Slide 19
[Fe(CO)4]2 Fe(2) d10 thus 10 +2(4) = 18 Fe2(CO)9 Fe(0) d8 thus: 2 metals 2(8) + CO ligands 9(2) MM bond 2 Total = 36 can view bridging CO; as 3 center 2 electron bond
OC OC OC Fe
O C C Fe O C O
CO CO CO
Slide 20
p10
04-Ktt212-18elect-09.pptx
5-Sep-10
Slide 21
Ligands
H-, [alkyl]- e.g. ([CH3]-, [C2H5]- dsb.), [aryl]- e.g. ([C6H5]-, [C6H5CH2]-, etc., CO, CS, CSe, PR3, P(OP)3, SbR3, F-, Cl-, Br-, I-, carbene(=CR2), alkene NO, NS, carbienes(CR) Dienes, [-allyl]-, [C5H5]-, [C4H4]2-, [C7H7]+ arene(C6H6, C6H5CO2CH3, etc.) [C3H3]3teraenes(C8H8) [C8H8]22
No. of electrons donated
3 4 6 8 10
Slide 22
p11
04-Ktt212-18elect-09.pptx
5-Sep-10
Slide 23
OC OC OC Fe OC
O C Fe
CO CO CO H
Fe = 8 e3 terminal CO = 6 eFe-Fe = 1 e1 H = 1 e2 CO = 2eTotal = 18 e-
Ph3 P Ni Ni P Ph3
Ni = 10 e5 -C5H5 = 5 e2-PPh3 = 3 Total = 18 eSlide 24
p12
04-Ktt212-18elect-09.pptx
5-Sep-10
Slide 25
Cr OC OC
Cr = 6 e6 -C6H6 = 6 e3 CO = 3*2 = 6 eTotal = 18 e-
OC Rh OC
Cl Rh Cl
CO
CO
CO
Rh = 9 e-Cl (1 e- Rh# 2 e- Rh##) = 3 e2CO = 4 eTotal = 16 e-
Slide 26
p13
04-Ktt212-18elect-09.pptx
5-Sep-10
OC OC OC Fe OC
O C Fe
CO CO CO H
Fe = 8 e3 terminal CO = 6 eFe-Fe = 1 e1 H = 1 e2 CO = 2eTotal = 18 eSlide 27
p14
You might also like
- Task 1 53 BP 19 RP F 0.35849 From Physical ChemistryDocument12 pagesTask 1 53 BP 19 RP F 0.35849 From Physical ChemistrysyavinaNo ratings yet
- Bioinorganic Handout PDFDocument63 pagesBioinorganic Handout PDFWwJd HeavenNo ratings yet
- PMR Spectroscopy: Solved Problems Volume : IIFrom EverandPMR Spectroscopy: Solved Problems Volume : IIRating: 5 out of 5 stars5/5 (3)
- PMHDocument1 pagePMHMohammed AltahirNo ratings yet
- Chapter 1Document67 pagesChapter 1Fitriani SariNo ratings yet
- CH6 InCh3101Document64 pagesCH6 InCh3101Fasil ManNo ratings yet
- Lecture 1Document11 pagesLecture 1Fang GaoNo ratings yet
- Silver BromideDocument9 pagesSilver BromideEric MonsalveNo ratings yet
- Aluminium Isopropoxide ReagentDocument5 pagesAluminium Isopropoxide ReagentSomu Yashawant ChaudhariNo ratings yet
- Tema 2Document5 pagesTema 2Tenten Higurashi Vi BritanniaNo ratings yet
- Pearson's Classification of Lewis Acids and Lewis Bases Into Hard and Soft - Acids and BasesDocument5 pagesPearson's Classification of Lewis Acids and Lewis Bases Into Hard and Soft - Acids and BasesThantea ChhakchhuakNo ratings yet
- Catalytic Oxidation of VOCs - ManuelaDocument31 pagesCatalytic Oxidation of VOCs - ManuelaSantiago Sánchez GómezNo ratings yet
- Reactions of Metal ComplexesDocument25 pagesReactions of Metal ComplexesNuansak3No ratings yet
- Alkane Nomenclature, Conformations, and SynthesisDocument41 pagesAlkane Nomenclature, Conformations, and SynthesisDenisse BadiolaNo ratings yet
- Temperature Programmed Desorption TPDDocument18 pagesTemperature Programmed Desorption TPDyiyiNo ratings yet
- BT tuần 2Document2 pagesBT tuần 2Đặng NhungNo ratings yet
- Enu Tour1 TaskDocument9 pagesEnu Tour1 TaskĐinh Đại VũNo ratings yet
- 01 1350977450 79497 PDFDocument83 pages01 1350977450 79497 PDFArya ChowdhuryNo ratings yet
- 8 Robinson AnnulationDocument21 pages8 Robinson AnnulationManish MeenaNo ratings yet
- Chapter18 Answer KeyDocument6 pagesChapter18 Answer KeyRavindra KempaiahNo ratings yet
- 1 ElectrochemistryTheoryDocument29 pages1 ElectrochemistryTheoryEdon Bedi100% (1)
- Alkene and Alkyne - by Resonance PDFDocument45 pagesAlkene and Alkyne - by Resonance PDFPrasad Yarra100% (1)
- Electrochemistry ExplainedDocument51 pagesElectrochemistry ExplainedManoj50% (2)
- Sigmatropic ReactionDocument14 pagesSigmatropic ReactionAatir HashmiNo ratings yet
- Giao Trinh Hoa Vo Co - Hay - NhatDocument194 pagesGiao Trinh Hoa Vo Co - Hay - NhatNguyễnn Linhh ChiiNo ratings yet
- The Chemistry NH2 NO NO2 Related GroupsDocument1,423 pagesThe Chemistry NH2 NO NO2 Related Groupsramik100% (1)
- 19 Petrucci10e CSMDocument52 pages19 Petrucci10e CSMPhạm Hoàng NamNo ratings yet
- 2016 Chimie Internationala Proba Teoretica SubiectebaremeDocument51 pages2016 Chimie Internationala Proba Teoretica SubiectebaremeCristinaNo ratings yet
- Oxidation Reduction Reactions ExplainedDocument21 pagesOxidation Reduction Reactions ExplainedKaroline UhlemannNo ratings yet
- German Problems 2014 Thu Nghiem 1Document447 pagesGerman Problems 2014 Thu Nghiem 1Hảo ĐặngNo ratings yet
- Chapter 11Document24 pagesChapter 11Biotechnology IIUM Kuantan100% (2)
- International Chemistry Olympiad SyllabusDocument12 pagesInternational Chemistry Olympiad SyllabuskyzzzNo ratings yet
- Group 1 - ETHYLBENZENE PRODUCTIONDocument7 pagesGroup 1 - ETHYLBENZENE PRODUCTIONQuỳnh Như PhạmNo ratings yet
- Organic Chemistry IR & NMR Practice ProblemsDocument12 pagesOrganic Chemistry IR & NMR Practice ProblemsJustin BuiNo ratings yet
- Notes Chapter 8 Transition ChemistryDocument17 pagesNotes Chapter 8 Transition ChemistryGauravRajNo ratings yet
- 36th Austrian Chemistry Olympiad National CompetitionDocument13 pages36th Austrian Chemistry Olympiad National CompetitionGerel BayrmagnaiNo ratings yet
- Real InDictionary of Chemistry 4 BookDocument33 pagesReal InDictionary of Chemistry 4 Booktire farrokhzadNo ratings yet
- Chapter 6-Alkyl Halides Nucleophilic Substitution: You May Have Drawn The Other Enantiomer. Either Is CorrectDocument23 pagesChapter 6-Alkyl Halides Nucleophilic Substitution: You May Have Drawn The Other Enantiomer. Either Is Correct張湧浩No ratings yet
- Aromaticity PDFDocument9 pagesAromaticity PDFabyssabhi100% (2)
- Hop Chat HC Don Chuc Tap 1 PGS TS Truong The KiDocument322 pagesHop Chat HC Don Chuc Tap 1 PGS TS Truong The KiKen PhanNo ratings yet
- Complexation and Precipitation Reactions and TitrationsDocument53 pagesComplexation and Precipitation Reactions and TitrationsDivya TripathyNo ratings yet
- Retro Synthetic Analysis GuidelinesDocument12 pagesRetro Synthetic Analysis GuidelinesaukidoNo ratings yet
- Hyper ConjugationDocument29 pagesHyper ConjugationDargorlethNo ratings yet
- OXAZOLEthiazolimidazoleDocument7 pagesOXAZOLEthiazolimidazole아미르No ratings yet
- ElectrochemistryDocument215 pagesElectrochemistryvisalNo ratings yet
- Hard/Soft Reactivity PrinciplesDocument1 pageHard/Soft Reactivity PrinciplesThomas BægelNo ratings yet
- HSAB TheoryDocument11 pagesHSAB TheoryMahesh Babu SriNo ratings yet
- Surface and Colloid ChemistryDocument8 pagesSurface and Colloid ChemistryLangmuir Hinshelwood Hougen WatsonNo ratings yet
- PhotochemistryDocument24 pagesPhotochemistryVijay PradhanNo ratings yet
- Kinetics Aspects Inorganic ChemistryDocument28 pagesKinetics Aspects Inorganic ChemistryFirda SafitriNo ratings yet
- Organometallic ChemistryDocument14 pagesOrganometallic ChemistryReine G100% (1)
- 1 IntroductoryDocument45 pages1 IntroductoryTuhin Sahu100% (1)
- Chapter 19. Aldehydes and Ketones: Nucleophilic Addition ReactionsDocument98 pagesChapter 19. Aldehydes and Ketones: Nucleophilic Addition Reactions張湧浩No ratings yet
- Transition Metal ToxicityFrom EverandTransition Metal ToxicityG. W. RichterNo ratings yet
- Electroanalysis: Theory and Applications in Aqueous and Non-Aqueous Media and in Automated Chemical ControlFrom EverandElectroanalysis: Theory and Applications in Aqueous and Non-Aqueous Media and in Automated Chemical ControlNo ratings yet
- How To Integrate STS in 21st Century (MUS)Document4 pagesHow To Integrate STS in 21st Century (MUS)Ahmad MuslihinNo ratings yet
- 4d3n Ho Chi Minh 08-11 Dec 2013Document3 pages4d3n Ho Chi Minh 08-11 Dec 2013Ahmad MuslihinNo ratings yet
- ReadmeDocument9 pagesReadmesawalliyatisNo ratings yet
- ReadmeDocument9 pagesReadmesawalliyatisNo ratings yet
- Higher Algebra - Hall & KnightDocument593 pagesHigher Algebra - Hall & KnightRam Gollamudi100% (2)
- Higher Algebra - Hall & KnightDocument593 pagesHigher Algebra - Hall & KnightRam Gollamudi100% (2)
- Organic and Inorganic Chemistry BridgedDocument16 pagesOrganic and Inorganic Chemistry BridgedDeepak KapaNo ratings yet
- Organometallic ChemistryDocument24 pagesOrganometallic ChemistryArangaNo ratings yet
- Organometallic Chemistry: Historical BackgroundDocument82 pagesOrganometallic Chemistry: Historical BackgroundĐức ThànhNo ratings yet
- GATE-2000 Chemistry Question PaperDocument326 pagesGATE-2000 Chemistry Question PaperPreeti Singh100% (3)
- Organometallic Organometallic Chemistry Chemistry: Hapter HapterDocument82 pagesOrganometallic Organometallic Chemistry Chemistry: Hapter HapterJ S.TNo ratings yet
- HW 9Document5 pagesHW 9Suryakant Pandey0% (1)
- Lecture 1 OrganometallicsDocument19 pagesLecture 1 OrganometallicsAaron GrettonNo ratings yet
- ANO7A MO Electroncount 2018 PDFDocument42 pagesANO7A MO Electroncount 2018 PDFJelte de WitNo ratings yet
- OrganometallicsDocument9 pagesOrganometallicsadarsh abcdNo ratings yet
- Chm242 Midsem MergedDocument125 pagesChm242 Midsem MergedLekha PylaNo ratings yet
- Dec 2011 Csir NetDocument23 pagesDec 2011 Csir NetAamerNo ratings yet
- Tutorial 3 - Qustions and AnswersDocument3 pagesTutorial 3 - Qustions and AnswersDhruv RaiNo ratings yet
- M. Sc. Chemistry Revised Syllabus 2022Document48 pagesM. Sc. Chemistry Revised Syllabus 2022Nabamita DawnNo ratings yet
- CHE 33-Lecture-1Document30 pagesCHE 33-Lecture-1Arafat Ahmed SajibNo ratings yet
- Metallic Carbonyls and NitrilesDocument40 pagesMetallic Carbonyls and NitrilesSandipan Saha100% (1)
- Organotransition Metal ChemistryDocument196 pagesOrganotransition Metal ChemistryKarzanNo ratings yet
- Metallic Carbonyls and Nitrosols Preparation and PropertiesDocument26 pagesMetallic Carbonyls and Nitrosols Preparation and PropertiesPriya RajanNo ratings yet
- 6 2018 11 10!08 18 38 PMDocument9 pages6 2018 11 10!08 18 38 PMAlma Tyara SNo ratings yet
- chm676 Notes PDFDocument43 pageschm676 Notes PDFEustance Juan100% (1)
- Catalysis 5Document52 pagesCatalysis 5Demon SamNo ratings yet
- Transition Metal ComplexesDocument103 pagesTransition Metal Complexesidownloadbooksforstu100% (1)
- Introduction To Transition Metal ComplexesDocument46 pagesIntroduction To Transition Metal ComplexesmaqboolsnNo ratings yet
- Inorganic Chemistry Exam 20100621Document2 pagesInorganic Chemistry Exam 20100621曾鈞浩No ratings yet
- Chemistry of Transition MetalsDocument44 pagesChemistry of Transition MetalsAqilah MahabirNo ratings yet
- Introduction To Organometallic Chem 01-4Document21 pagesIntroduction To Organometallic Chem 01-4Aparna DuggiralaNo ratings yet
- OrganometallicsDocument53 pagesOrganometallicsSaman KadambNo ratings yet
- Lecture 20. An Introduction To Organometallic Chemistry: Benzene The Sandwich' Complex of CR (0), Which Is (CR (Benzene) )Document16 pagesLecture 20. An Introduction To Organometallic Chemistry: Benzene The Sandwich' Complex of CR (0), Which Is (CR (Benzene) )Hafizah RamliNo ratings yet
- JAM 2020 Chemistry - CyDocument21 pagesJAM 2020 Chemistry - CySubhasish PatraNo ratings yet
- Aid For Ferrocene AssignDocument32 pagesAid For Ferrocene AssignGuangyu XuNo ratings yet
- Electron Counting in Organometallic Chemistry: 1. The 18-Electron Rule Definition & RationalisationDocument11 pagesElectron Counting in Organometallic Chemistry: 1. The 18-Electron Rule Definition & Rationalisationgaurav100% (1)