Professional Documents
Culture Documents
Antiarrhythmic Drugs
Uploaded by
emiliow_1Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Antiarrhythmic Drugs
Uploaded by
emiliow_1Copyright:
Available Formats
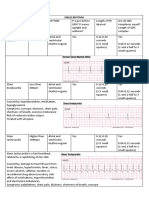
Table 2132.
ANTIARRHYTHMIC DRUGS (VAUGHAN WILLIAMS CLASSIFICATION)
DRUG
Class Ia
DOSAGE
TARGET LEVELS
SELECTED ADVERSE EFFECTS
COMMENTS
Uses: APB and VPB suppression, SVT and VT suppression, AF or atrial flutter, and VF suppression Drug should be used cautiously in patients with impaired LV function. Dosage should be decreased in patients with renal insufficiency. Adverse effects may contribute to nonadherence. If QRS interval widens (> 50% if initially < 120 msec or > 25% if initially > 120 msec) or if QTc interval is prolonged > 550 msec, infusion rate or dosage should be decreased or drug stopped. IV form is not available in the US. Sustained-release preparations obviate the need for frequent dosing. If QRS interval widens (> 50% if initially < 120 msec or > 25% if initially >120 msec) or if QTc interval is prolonged > 550 msec, infusion rate or dosage should be decreased or drug stopped. If QRS interval widens (> 50% if initially < 120 msec or > 25% if initially > 120 msec) or if QTc interval is prolonged > 550 msec, infusion rate or dosage should be decreased or drug stopped. To reduce toxicity risk, clinicians should reduce dosage or infusion rate to 2 mg/min after 24 h.
Disopyramide IV: Initially, 27.5 g/mL Anticholinergic 1.5 mg/kg over effects (urinary re> 5 min foltention, glaucoma, lowed by an dry mouth, blurred infusion of vision, intestinal 0.4 mg/kg/h upset), hypoglyceOral immediatemia, torsades de release: 100 or pointes VT; nega150 mg q 6 h tive inotropic effects Oral controlled(which may worsen release: 200 or heart failure or 300 mg q 12 h hypotension)
Procainamide
IV: 1015 mg/ kg bolus at 2550 mg/ min, followed by a constant IV infusion of 14 mg/min Oral: 250625 mg (rarely, up to 1 g) q 3 or 4h Oral: 200400 mg q 46 h
48 g/mL
Quinidine
26 g/mL
Hypotension (with IV infusion), serologic abnormalities (especially ANA) in almost 100% taking drug for > 12 mo, drug-induced lupus (arthralgia, fever, pleural effusions) in 1520%, agranulocytosis in < 1%, torsades de pointes VT Diarrhea, colic, flatulence, fever, thrombocytopenia, liver function abnormalities, torsades de pointes VT; overall adverse effect rate of 30%
Class Ib
Uses: Suppression of ventricular arrhythmias (VPB, VT, VF) IV: 100 mg over 25 g/L 2 min, followed by continuous infusion of 4 mg/min Tremor, seizures; if administration is too rapid, drowsiness, delirium, paresthesias;
Lidocaine
Table 2132. ANTIARRHYTHMIC DRUGS (VAUGHAN WILLIAMS CLASSIFICATION) (Continued )
DRUG DOSAGE TARGET LEVELS SELECTED ADVERSE EFFECTS COMMENTS
Lidocaine (contd)
Mexiletine
(2 mg/min in possibly increased patients > 65); risk of bradyar5 min after first rhythmias after dose, a 2nd acute MI 50-mg bolus is given Oral immediate- 0.52 g/mL Nausea, vomiting, tremor, seizures release: 100 250 mg q 8 h Oral slowrelease: 360 mg q 12 h IV: 2 mg/kg at 25 mg/min, followed by 250mg infusion over 1 h, 250-mg infusion over next 2 h, and maintenance infusion of 0.5 mg/min
Extensive first-pass hepatic metabolism occurs.
Oral slow-release and IV forms are not available in the US.
Class Ic
Flecainide
Propafenone
Uses: APB and VPB suppression, SVT and VT suppression, AF or atrial flutter, and VF suppression Oral: 100 mg 0.21 g/mL Occasionally, IV form is not available q 8 or 12 h blurred vision and in US. IV: 12 mg/kg paresthesias If QRS complex widens over 10 min (> 50% if initially < 120 msec and > 25% if initially > 120 msec), dose must be decreased or drug stopped. 0.11.0 g/ -blocking activity, Pharmacokinetics are Oral: Initially, mL 150 mg tid, possible worsening nonlinear; increases titrated up to of reactive airway in dose should not 150300 mg tid disorders; occaexceed 50% of IV: 2-mg/kg sionally GI upset previous dose. bolus, followed Bioavailability and by 2 mg/min protein binding infusion vary; drug has saturable first-pass metabolism. IV form is not available in the US. Uses: Supraventricular tachyarrhythmias (APB, ST, SVT, AF, atrial flutter) and ventricular arrhythmias (often in a supportive role) Oral: 50100 mg once/day Oral: Initially, 6.25 mg bid, followed by titration to 25 mg bid -Blocker levels not measured; dose adjusted to reduce heart rate by > 25% Typically for -blockers are contrain-blockers, GI disdicated in patients with turbances, insomnia, bronchospastic airway nightmares, lethargy, disorders. erectile dysfunction, possible AV block in patients with AV node dysfunction
Table continues on the following page.
Class II
(-blockers) Atenolol Carvedilol
Table 2132. ANTIARRHYTHMIC DRUGS (VAUGHAN WILLIAMS CLASSIFICATION) (Continued )
DRUG
Class II
DOSAGE
TARGET LEVELS
SELECTED ADVERSE EFFECTS
COMMENTS
(-blockers) (contd) Acebutolol Betaxolol Bisoprolol Esmolol Metoprolol Oral: 200 mg bid Oral: 20 mg once/day Oral: 510 mg once/day IV: 50200 g/ kg/min Oral: 50100 mg bid IV: 5 mg q 5 min up to 15 mg Oral: 6080 mg once/day Oral: 1030 mg tid or qid IV: 13 mg (may repeat once after 5 min if needed) Oral: 1020 mg bid Uses: Any tachyarrhythmia except torsades de pointes VT
Nadolol Propranolol
Timolol
Class III
(membranestabilizing drugs) Amiodarone
Oral: 6001200 12.5 g/mL Pulmonary fibrosis (in Drug has noncompetitive mg/day for up to 5% of patients -blocking, Ca channel blocking, and Na chan710 days, then treated for > 5 yr), 400 mg/day which may be fatal; nel blocking effects, with a long delay in for 3 wk, folQTc prolongation; onset of action. lowed by a torsades de pointes maintenance VT (rare); bradycar- By prolonging refractoriness, drug may cause dose (ideally, dia; gray or blue 200 mg/day) discoloration of sun- homogeneous conditions of repolarization exposed skin; sun IV: 150450 mg throughout the heart. sensitivity; hepatic over 16 h (deabnormalities; peri- IV form can be used for pending on urconversion. pheral neuropathy; gency), folcorneal microdeposlowed by a its (in almost all maintenance treated patients), usudose of 0.52.0 ally without serious mg/min visual effects and reversed by stopping the drug; changes in thyroid function; slow clearance possibly prolonging adverse effects
Table continues on the following page.
Table 2132. ANTIARRHYTHMIC DRUGS (VAUGHAN WILLIAMS CLASSIFICATION) (Continued )
DRUG DOSAGE TARGET LEVELS SELECTED ADVERSE EFFECTS COMMENTS
Azimilide Bretylium*
Dofetilide
Ibutilide
Sotalol
Oral: 100200 mg once/day IV: Initially, 5 mg/kg, followed by 12 mg/min as a constant infusion IM: Initially, 510 mg/kg, which may be repeated to a total dose of 30 mg/kg IM maintenance dose of 5 mg/ kg q 68 h IV: 2.54 g/mL Oral: 500 g bid if CrCl > 60 mL/min; 250 g bid if CrCl is 4060 mL/min; 125 g bid if CrCl is 2040 mL/min IV: For patients 60 kg, 1 mg infusion or, for patients < 60 kg, 0.01 mg/kg over 10 min, with dose repeated after 10 min if the first infusion is unsuccessful Oral: 80160 mg q 12 h IV: 10 mg over 12 min
2001000 ng/mL 0.82.4 g/ mL
Torsades de pointes VT Hypotension
Drug has class II properties. Effects may be delayed 1020 min.
N/A
Torsades de pointes VT
Drug is contraindicated if QTc > 440 msec or if CrCl < 20 mL/min.
N/A
Torsades de pointes VT (in 2%)
Drug is used to terminate AF (success rate, about 40%) and atrial flutter (success rate, about 65%).
0.54 g/mL Similar to class II; possible depressed left ventricular function and torsades de pointes VT
Racemic [D-L] form has class II (-blocking) properties, [D] form does not. Both forms have class III activity. Only racemic sotalol is available for clinical use. Drug should not be used in patients with renal insufficiency.
Table continues on the following page.
Table 2132. ANTIARRHYTHMIC DRUGS (VAUGHAN WILLIAMS CLASSIFICATION) (Continued )
DRUG
Class IV
DOSAGE
TARGET LEVELS
SELECTED ADVERSE EFFECTS
COMMENTS
(Ca channel blockers) Diltiazem
Uses: Termination of SVT and slowing of rapid AF or atrial flutter 0.10.4 g/ Oral slowmL release (diltiazem CD): 120 mg to 360 mg once/day IV: 515 mg/h for up to 24 h Oral: 40120 mg N/A tid or, for sustained-release form, 180 mg once/day to 240 mg bid IV: 515 mg over 10 min Oral prophylaxis: 40120 mg tid 6 mg rapid IV bolus, repeated twice at 12 mg if needed; flush bolus with additional 20 mL saline IV loading dose: 0.5 mg Oral maintenance dose: 0.1250.25 mg/day N/A
Possible precipitation of VF in patients with VT, negative inotropy
IV form is most commonly used to slow ventricular response rate to AF or atrial flutter.
Verapamil
Possible precipitation of VF in patients with VT, negative inotropy
IV form is used to terminate narrowcomplex tachycardias involving the AV node (success rate, almost 100% with 510 mg IV over 10 min).
Other antiarrhythmics
Adenosine
Transient dyspnea, chest discomfort, and flushing (in 3060%), transient bronchospasm
Digoxin
0.81.6 g/ mL
Anorexia, nausea, vomiting, and often serious arrhythmias (VPBs, VT, APBs, atrial tachycardia, 2nd-degree or 3rd-degree AV block, combinations of these arrhythmias)
Drug slows or blocks AV nodal conduction. Duration of action is extremely short. Contraindications include asthma and high-grade heart block. Dipyridamole potentiates effects. Contraindications include antegrade conduction over an accessory AV connection pathway (manifest Wolff-ParkinsonWhite syndrome) because if AF occurs, ventricular responses may be excessive (digoxin shortens refractory periods of the accessory connection).
*Availability uncertain. AF = atrial fibrillation; ANA = antinuclear antibody; APB = atrial premature beat; AV = atrioventricular; CrCl = creatinine clearance; LV = left ventricular; QTc = QT interval corrected for heart rate; SVT = supraventricular tachycardia; VF = ventricular fibrillation; VPB = ventricular premature beat; VT = ventricular tachycardia.
Copyright 2011 Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Whitehouse Station, N.J. U.S.A.
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- Day Trading MarginDocument6 pagesDay Trading Marginemiliow_1No ratings yet
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- 4 7 11 PsoriasisDocument8 pages4 7 11 Psoriasisemiliow_1No ratings yet
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Human Immunodeficiency Virus and AIDSDocument10 pagesHuman Immunodeficiency Virus and AIDSemiliow_1No ratings yet
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (894)
- Success For Teens Workbook PDFDocument35 pagesSuccess For Teens Workbook PDFSentenyi100% (1)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Karl Marx and MarxismDocument7 pagesKarl Marx and MarxismBenny Apriariska SyahraniNo ratings yet
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Pregnancy Category D Antiepileptic Drugs Weight Effects InteractionsDocument6 pagesPregnancy Category D Antiepileptic Drugs Weight Effects Interactionsemiliow_1No ratings yet
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Phenytoin Dosing GuidelinesDocument9 pagesPhenytoin Dosing Guidelinesemiliow_1No ratings yet
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Maxwell Mastermind GuidesDocument27 pagesMaxwell Mastermind Guidesemiliow_1No ratings yet
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- Evolution of Psychiatric Medications-1Document4 pagesEvolution of Psychiatric Medications-1emiliow_1100% (1)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- Thomas Paine's Common Sense Calls for American IndependenceDocument51 pagesThomas Paine's Common Sense Calls for American Independenceemiliow_1No ratings yet
- Socrates Theoretical Analysis and Thought ProcessDocument11 pagesSocrates Theoretical Analysis and Thought Processemiliow_1No ratings yet
- Schizophrenia and Mental Disorders in Patients An The TreatmentDocument7 pagesSchizophrenia and Mental Disorders in Patients An The Treatmentemiliow_1No ratings yet
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Combo Medication To Help Patients Overcome IllnessDocument16 pagesCombo Medication To Help Patients Overcome Illnessemiliow_1No ratings yet
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- Heart Failure: Non-Pharmacologic TherapyDocument5 pagesHeart Failure: Non-Pharmacologic Therapyemiliow_1No ratings yet
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- Latin Route WordsDocument38 pagesLatin Route Wordsemiliow_1No ratings yet
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Asthma GuideDocument2 pagesAsthma GuideJearome TaboyNo ratings yet
- Management of Oral Anticoagulant Txt22S.fullDocument19 pagesManagement of Oral Anticoagulant Txt22S.fullemiliow_1No ratings yet
- Asthma GuideDocument2 pagesAsthma GuideJearome TaboyNo ratings yet
- Guia Accp 2012 Sobre AntitromboticosDocument41 pagesGuia Accp 2012 Sobre AntitromboticosrhymescsfNo ratings yet
- Training ManualDocument41 pagesTraining Manualemiliow_1100% (3)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- GetBackgroundReport DoDocument1 pageGetBackgroundReport Doemiliow_1No ratings yet
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- Endrocrine QuestnsDocument5 pagesEndrocrine Questnsemiliow_1No ratings yet
- Kinetics Exam 3Document79 pagesKinetics Exam 3emiliow_1No ratings yet
- Antimicrobial ReviewDocument57 pagesAntimicrobial Reviewemiliow_1No ratings yet
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- Antimicrobial ReviewDocument57 pagesAntimicrobial Reviewemiliow_1No ratings yet
- Citation 1Document1 pageCitation 1emiliow_1No ratings yet
- Pharmacist's Manual - An Information Outline of The Controlled Substances Act - 2010Document85 pagesPharmacist's Manual - An Information Outline of The Controlled Substances Act - 2010James LindonNo ratings yet
- Special Resource: Drugs To Be Used With A Filter For Preparation And/or AdministrationDocument5 pagesSpecial Resource: Drugs To Be Used With A Filter For Preparation And/or Administrationemiliow_1No ratings yet
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Case Report ExampleDocument7 pagesCase Report Exampleemiliow_1No ratings yet
- HypomagnesemiaDocument3 pagesHypomagnesemiaapi-3712326No ratings yet
- Sample Nursing Exams - Answer & RationaleDocument58 pagesSample Nursing Exams - Answer & RationaleKars AtacadorNo ratings yet
- The RX Files: QT Prolongation and Torsades de Pointes: Drugs and Sudden DeathDocument2 pagesThe RX Files: QT Prolongation and Torsades de Pointes: Drugs and Sudden DeathRahul RaiNo ratings yet
- Manual of Emergency Medicine, 6e (May 25, 2011) - (1608312496) - (LWW) PDFDocument719 pagesManual of Emergency Medicine, 6e (May 25, 2011) - (1608312496) - (LWW) PDFpopoying100% (2)
- Antiarrhythmic AgentsDocument45 pagesAntiarrhythmic AgentsSoh Kae SiangNo ratings yet
- Emp SyncopeDocument20 pagesEmp SyncopeWilmer JimenezNo ratings yet
- NCLEX: From UworldDocument26 pagesNCLEX: From UworldOlgaKNo ratings yet
- Pharmacology Misbah PDFDocument238 pagesPharmacology Misbah PDFRiham Khamis86% (7)
- Guias Hipertension 2023 Esc BDocument14 pagesGuias Hipertension 2023 Esc Bmiguel contrerasNo ratings yet
- Amiodarone InjDocument2 pagesAmiodarone InjasdwasdNo ratings yet
- Cardiac Arrhythmias Cardiac ArrhythmiasDocument29 pagesCardiac Arrhythmias Cardiac ArrhythmiasgowthamNo ratings yet
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Generic Name Brand Name Generic Name Brand Name Generic Name Brand NameDocument3 pagesGeneric Name Brand Name Generic Name Brand Name Generic Name Brand NameIulia TodericiNo ratings yet
- Important Causes of Sudden Cardiac DeathDocument2 pagesImportant Causes of Sudden Cardiac DeathTausif AbbasNo ratings yet
- Antiarrhythmic Drug GuideDocument42 pagesAntiarrhythmic Drug GuideRamadi PrameelaNo ratings yet
- Vitamins, Supplements, Herbal Medicines, and ArrhythmiasDocument12 pagesVitamins, Supplements, Herbal Medicines, and ArrhythmiashyntnenNo ratings yet
- Cardiac ArrhythmiasDocument60 pagesCardiac ArrhythmiasMelissa Monique Peart-YatesNo ratings yet
- ... & More: ECG ChallengeDocument2 pages... & More: ECG ChallengehameunjungNo ratings yet
- Bradycardia and Tachycardia Treatment OptionsDocument31 pagesBradycardia and Tachycardia Treatment OptionscmirceaNo ratings yet
- Journal of Arrhythmia - 2022 - Ono - JCS JHRS 2020 Guideline On Pharmacotherapy of Cardiac ArrhythmiasDocument141 pagesJournal of Arrhythmia - 2022 - Ono - JCS JHRS 2020 Guideline On Pharmacotherapy of Cardiac ArrhythmiastochtliNo ratings yet
- Approach To Ventricular ArrhythmiasDocument18 pagesApproach To Ventricular ArrhythmiasDavid CruzNo ratings yet
- ECG Arrhythmia GuideDocument5 pagesECG Arrhythmia GuideMasarrah AlchiNo ratings yet
- Acls Drug OverviewDocument2 pagesAcls Drug OverviewBruce Abramowitz100% (1)
- Draft Information Package Leaflet Regarding Polysorbates Used Excipients Medicinal Products Human - enDocument42 pagesDraft Information Package Leaflet Regarding Polysorbates Used Excipients Medicinal Products Human - enabhi200291No ratings yet
- Trousseau Sign - StatPearls - NCBI BookshelfDocument4 pagesTrousseau Sign - StatPearls - NCBI BookshelfVictoria NatashaNo ratings yet
- Cardiac Surgery - Postoperative ArrhythmiasDocument9 pagesCardiac Surgery - Postoperative ArrhythmiaswanariaNo ratings yet
- ACLS DrugsDocument16 pagesACLS Drugstostc100% (1)
- UW - Cardiovascular - Educational ObjectivesDocument52 pagesUW - Cardiovascular - Educational ObjectivesUsama BilalNo ratings yet
- Pharmacology of Antiarrhythmic DrugsDocument51 pagesPharmacology of Antiarrhythmic DrugsselflessdoctorNo ratings yet
- PracticeExam 2 AnsDocument51 pagesPracticeExam 2 AnsBehrouz YariNo ratings yet
- FDA Powerpoint Presentation: Torsades de Pointes and QT ProlongationDocument51 pagesFDA Powerpoint Presentation: Torsades de Pointes and QT ProlongationByron Harding100% (1)