Professional Documents
Culture Documents
By V. K. B B An, B.S., T. G. Kaufman, B.S., H. L - MX - , B.S. and R. J. T - Aczuk, M.S.
Uploaded by
techkasambaOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
By V. K. B B An, B.S., T. G. Kaufman, B.S., H. L - MX - , B.S. and R. J. T - Aczuk, M.S.
Uploaded by
techkasambaCopyright:
Available Formats
J. soc. cos. CHEM.
15, 473-483 (1964)
SOME
USES
AND
APPLICATIONS IN COSMETIC
OF AND
POLYGLYCEROL
ESTERS
PHARMACEUTICAL
PREPARATIONS
By V. K. B^B^AN, B.S., T. G. KAufMaN, B.S., H. L.Mx, B.S. and R. J. TaczuK, M.S.*
Presented November 6, 1963, New York Chapter
ABSTR>
Polyllycerol esters as a class of emulsifiers having a wide range of hydrophilic-lipophilic characteristics have been prepared and characterized. Their properties and characteristics are discussed and compared with those of other groups and classes of emulsifiers. Applications and uses of the polyllycerol esters are discussed, with particular emphasis on their use as emulsifiers. It is shown that some esters are especially suitable for use and give the most stable W/O emulsions with certain oils. In addition, the results of microbiological tests are given, indicating that hydrophilic polyllycerol esters do not
interfere with the bacteriostatic action of G-11.
INTRODUCTION
The large numberof emulsifiers that is currentlyavailableto the cosmeticand pharmaceutical industriesis generallydivided into four classes: anionic,nonionic, cationicand amphoteric. Typical examples of anionicemulsifiers are materialssuchas soaps, sodiumlauryl isethionate, sulfatedoils,etc. Anionicsurfactants have the advantages of beinguseful in small concentrations; however,they are sensitive to the presence of other ions,acidsand cationicemulsifiers (1). In addition,their internal
use is limited.
Typical examples of nonionic emulsifiers are materials suchas polyethylene glycol esters, sorbitan and polyoxyethylene sorbitan esters, ethoxylated fatty alcohols, alkanolamides, etc. Nonionicemulsifiers de* Drew Chemical Corp., Boonton, N.J.
473
474
JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS
pend chieflyupon ether linkagesand hydroxylgroupsto create their hydrophilicaction. Sincethey do not ionize, they are claimedto be the least irritating of emulsifiers(1). However, nonionicsand especially thosethat are ethoxylatedhave beenreportedby many authorsto inactivate preservatives (2-7). The most frequently encounteredcationic emulsifiersare quaternary ammonium compoundssuch as stearyl dimethyl benzyl ammonium chloride. Cationic emulsifiersare generallystable only at neutral and acid pH's, are considered to be the most irritating class of surfactants,and are not allowedfor internal use (8). In addition, there are relatively few
cationicedible and W/O emulsifiers available. Thus, there is a definiteneedfor a new class of emulsifiers which is edible, coversa wide rangeof hydrophilic-lipophilic propertiesand is nonethoxy-
late& This hasbecome particularlyimportantwith the establishment of stringent controls on food,drugandcosmetic ingredients andadditives.
THE POLYGLYCEROL ESTERS
Although polyglycerolestershave been known for over thirty years, very little hasbeen writtenandevenless usehasbeen madeof their unique physicaland chemicalproperties. The major reasonfor this neglectis, perhaps,the difficultiesof preparing materials with reproducibleresults (9). In recentyears,suitablemethods have beendeveloped, and patents for the preparationand analysisof the polyglycerols and polyglycerol esters arenowpending(10). The statusof thepolyglycerol esters canbesummed up asfollows:
1. Polyglycerol esters areprepared fromglycerine, fats andoilsandfatty
acids.
2. Functionalitycan be built into the structureas desired, rangingfrom complete oil solubilityto completewater solubility. 3. Tailor-made emulsifiers from polyglycerols can be made to suit or fit the needsof the cosmeticand pharmaceutical industries. 4. A process hasbeendeveloped in our laboratories whichyieldsproducts of goodcolor,odor and flavor. 5. They are readilyavailable,and the costis within reason. 6. The humanbodyis ableto utilizethe polyglycerol derivatives just like
the common fats and oils (12).
7. No accumulation or toxic effect couldbe found in the usageof poly-
glycerol esters evenwhenusedasthe sole source of fat. As products that couldachieve low or high molecular weight,solidor liquidconsistency, wateroroilsolubility in thecomplete absence oftoxicity, thesepolyglycerol estersbecamea welcome addition to the line of acceptable emulsifiers oncethe F.D.A. gavethe necessary clearance to their
use.
POLYGLCEROL
ESTERS
IN
PHARMACEUTICAL
PREPARATIONS
475
TABLE I--SOME
PHYSICAt, AND CHEMICAL CHAR.ACTEKISTICSOF POLYGLYCEKOLS
Compound
Glycerol Di-glycerol
Molecular Weight
92 166
Number of OH Groups
3 4
Calculated OH Value
1830 1352
Found OH Value
1828 1320
Specific Gravity
1.24
Tri-glycerol
Tetra-glycerol Penta-glycerol Hepta-glycerol Octa-glycerol Nona-glycerol
240
314 388 536 610 684
l 169
1071 1012 941 920 903
1166
972 951 903
l'.
...
Hexa-glycerol 462
Deca-glycerol 758
6 7
970
1010
888
1082 1028
1"2'4
... ...
9 10 11
12
880
l'
Polyglycerols are prepared by the polymerizationof glycerineunder alkaline conditions. The processhas been recently developed, and a patent is pendingwith the U.S. Patent OfFice. The controlledpolymerization of glycerolto yield a specific molecularweight polymer produces water white to pale yellow productswith pleasantodor and flavor. The polyglycerols thus preparedare then esterifiedwith specific fatty acidsor subjectedto alcoholysis or ester exchange to preparea mixed fatty acid ester. Thus, a hexaglycerol monostearate may best be made by reacting hexaglycerol with stearicacid, while a hexaglycerol coconutoil ester may best be prepared by the alcoholysis of coconut oil with hexaglycerol. Investigationsthus far indicate that the polymerizationof glycerol is progressing in a straightline mannerwithout cyclization or ring formation. Although Markley (11) indicatescyclic structure formation, we have not been able to identify the presence of any ring structuresin our product. Work is continuingto shedlight on the mechanism of this reaction, but it appears,at present,that the polymerizationgives rise to the type of chaingrowthwhichis shownbelow:
OH HC H OH H H OH H C--C-4)--C--C--C---O--C--C H OH OH C--H
The polymerizationcan continuein this manner, with the terminal hydroxylgroups formingetherlinkages by eliminationof water.
CHEMICAL AND PHYSICAL PROPERTIES
The polyglycerolshave the characteristics normally associatedwith polyols. They are water-soluble in all proportionsand have excellent emollientand humectantproperties. They differ from glycerolmainly in that the viscosityincreases as the chain lengthdoes. Table I shows some
476
JOURNAL OF THE SOCIETY OF COSMETIC
CHEMISTS
of thechemical properties of polyglycerols, starting with thesingle glycerol molecule and goingup to the decaglycerol molecule which contains10 moles of glycerol. It should be notedthat therewill always be two more hydroxyl groups thantherearemoles of glycerol. These figures show the numerical increase in thehydroxyl groups in each polyglycerol asthepolymer is increased in sizeandmolecular weight. Also,the calculated hydroxylvaluesand the actualhydroxyl valuesobtained by analysis are given to demonstrate the correlation between hydroxylvalueandmolecular weight.
It has beenpossible to preparepolymers from diglycerol(2 molesof glycerol) to tricontaglycerol (30 moles of glycerol) in the initial stages of polymerization.The polyglycerols are high-molecular weight, watersoluble fluidswhichmay be usedasthickening agents, humectants, chemicalintermediates andfor otherpurposes.
Oneor moreof the hydroxyl groups present in a polyglycerol molecule may be esterified;thus,since decaglycerol contains 12 hydroxyl groups, any esterfrom monoto dodeca may be prepared. Sinceany number of fatty acids may be used in the esterification, the number of possible polyglycerolestersbecomes virtually unlimited. These polyglycerol esters may be liquidto waxy; saturated or unsaturated; low to highmolecular weight; and hydrophilic or lipophilic, depending on the numberof hydroxylgroups that arereacted with the fatty acids and/oroilsin question. Polyglycerol esters ranging fromcomplete oil solubilityto complete water solubilitymay be prepared,with the possibilityof many intermediate properties between these two extremes. Therefore, it becomes possible to synthesize an entireclass of emulsifiers, tailor-made to perform anyspecific function. TableII illustrates some of the chemical andphysical properties of a selected list of the polyglycerol esters, and Table III shows their
solubilities in various solvents.
Regardless of the molecular weightof the polyglycerol and regardless of the typeof fatty acidor amount of such fatty acidpresent in thepolyglycerol ester, feeding studies (12) have indicated that such esters are
completely nontoxic and are degraded fully by the bodyto yieldglycerol and the fatty acid. Why a three-carbon ether linkageshould be utilized by the bodywhilea two-carbon etherlinkage of thepolyoxyethylene productsshould not be utilizedis not clearlyunderstood.The repeated resultsof testing,however,leave no questionthat this is true. Basedon thesefeedingstudies,which were conductedwith saturated and unsatu-
ratedesters of polyglycerol ranging from twoto thirty moles of glycerine, the F.D.A. not only gaveapproval on the useof these polyglycerol esters in foodbut alsoplacedno limits on the amounts that couldbe used(13). This F.D.A. approval currentlycovers esters up to and including the decaglycerol esters of the fatty acids derivedfrom corn,cottonseed, lard,
POLYGLYCEROI. ESTERS IN PHARMACEUTICAL
PREPARATIONS
477
478
JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS
POLYGLYCEROL
ESTERS
IN
PHARMACEUTICAL
PREPARATIONS
479
palm fruit, peanut, safflower, sesame and soybeanoils, and tallow. Further approvals areexpected in the nearfuture.
APPLICATIONS
1. Since a number of theseestersare lipophilic in nature, they should constitutea family of W/O emulsifiers with a particularly wide range of properties. The literature containsmany references to work on the selectionof suitable O/W emulsifiersfor various oils (14-15), but apparently little suchwork has beendoneon W/O emulsions;in fact, difFiculties had been encounteredin preparing stable W/O emulsionswith vegetableoils as the externalphase (16). It seemed important, therefore, to test a series of the polyglycerol esters asW/O emulsifiers for various oils to determine the mostsuitable polyglycerol esterfor emulsification of
each oil.
The following oils were used: two grades of mineral oil; isopropyl
myristate; peanut oil; Neobee MSS*; NeobeeO*; corn oil; Robanent;
and lanolin oils.
The tabulatedmaterialswereemployed asemulsifiers:
1. A 50/50 mixture of decaglycerol dodecaoleate and decaglycerol decaoleate.
2. A 50/50 mixture of decaglycerol decastearate and decaglycerol decaoleate.
3. 4. 5. 6. 7.
Decaglycerol decaoleate. Decaglycerol hexaoleate. Decaglycerol octaoleate. Decaglyceroltetraoleate. Sorbitansesquioleate (as control).
All emulsions were preparedin 500 g. quantities,using 65% oil, 25% water and 10% emulsifier. The oil (containing the emulsifier) and the water were heatedseparatelyto 78 C, and the water was slowly addedto the oil phaseusingrapid agitation,mixingbeingcontinued for 30 minutes. The emulsions were observed initially, after one and three days, after one
and two weeks and after one month.
Figure1 shows theseries using lightmineraloil astheexternal phase after intervalsof one day and one and two weeksrespectively. Figure 2 shows the equivalentseries for heavy mineral oil. It is noted that the greatest stability was obtained with decaglycerol tetraoleate (10-4-0). Similar resultswere obtainedwith isopropyl myristateand with Robane. Figure 3 showsa similar seriesusing Neobee O as the external phase. In this case, the beststabilitywasobtained with a blendof decaglycerol decaoleate
* Neobeeis Drew ChemicalCorporation's trademarkfor synthetictriglycerides. t Robaneis RobecoChemicals' Inc. trademarkfor hydrogenated squalene.
480
JOURNAl. OF THE SOCIETY OF COSMETIC
CHEMISTS
and decaglycerol decastearate (10-10-0/10-10-s); the secondbest stability was obtained with decaglycerol decaoleate(10-10-0) alone. Similar results wereobtainedwith NeobeeMS, peanutoil and cornoil. Basedon theseresults severalW/O emulsions which could be readily adaptedfor cosmetic or pharmaceutical usewereprepared. Theseformulations are shown in Table IV.
It has been possibleto prepare many excellent cosmeticcreams and lotionsby the useof theseesters. Decaglycerol monoesters, such as the
7-/' AV
IN.A.O/
LIGHTMINEAL OIL.i.
. .
-.'" ,,:; . :m; ...s. ......
Figure 1.--Stabilityof emulsions prepared Figure2.--Stability of emulsions prepared withlightmineral oil. with heavy mineral oil.
palmitate,stearate andoleate, havebeenfoundto be veryeffective hydrophilic emulsifiers, while decaglycerol tri- and tetra-esters,such as decaglycerol tristearate or decaglycerol tetraoleate, are excellent auxiliary emulsifiers. Two formulationsusing theseestersare includedin Table
IV.
2. Althougha great variety of nonionic O/W emulsifiers is currently available, thereare still some problems that occur in the useof nonionics,
POLYGLYCEROL
ESTERS IN PHARMACEUTICAL
PREPARATIONS
481
......
.''."c":' "" '..>":.:.'.:.." .'.:::.:...:V.%.:...: .....................
.'::.
0o-0 --o
............... .:::...... ....
' NEOBE.E 0
. .
: ' :. : .. ....
.... =.:
.'-
.: .......
..- .o:--..2%'.:...::=: '.: ...... ..-
...........
'..... ' :':::": ...
" ', ".4.: . ..... . ....... . ....
Figure &--Stability of emulsions preparedwith NeobeeO.
primarily those containingpolyoxyethylene. One of these problemsis the interferencethat has been frequently reported between ethoxylated emulsifiers and bacteriostats of the bisphenoltype, such as Hexachlorophene,* or of the alkyl parahydroxybenzoates (2-7). It was, therefore, decidedto use zone of inhibition teststo compareseveral of the polyglycerol esterswith other nonionic surfactants. To date, these tests have been run using a 0.5% alcoholicsolution of Hexachlorophene with eight different emulsifiersat concentrations up to 2%. Test organismsinclude two gram negative bacteria(Escherichia coli and Pseudomonas aeruginosa) and a mold (/Ispergillusniger). Tests usinggram positiveorganisms will bepublished at a later date. The resultsin Table V suggest that the polyglycerolestersshow promiseof being lesslikely to interferewith Hexachlorophene than other nonionic emulsifiers. Further experimentsto substantiate theseresultsare now beingundertaken. 3. Many other applications of the polyglycerolestersare possibleas a result of their unique chemicaland physical properties. Thus, the stearates can be used as gellingagentsfor mineral oils, vegetable oils and glycols. For instance,decaglycerol monostearatewill gel mineral oil, and the resulting gel can be readily rinsed with water. Materials such as decaglycerol tristearateand polyglycerol oleatescan be usedto formulate
* G-11--Sindar Corporation, Delawanna,N.J. (7).
482
JOURNAL OF THE SOCIETY OF COSMETIC
TABLE VI--CoMPOSlTION
CHEMISTS
OF EMULSIONS PREPARED WITH POLYGLYCEROLESTEKS
Formula #1 (Vegetable Oil Emulsion) Neobee 54* .................... 6%
Decaglycerol decaoleate ......... Decaglycerol decastearate ........
Neobee t .................... Water ........................
5%
45% 39%
Formula #2 (Peanut Oil Emulsion) Beeswax:..................... Neobee 54* .................... 3% Magnesium stearate............ 1% Decaglycerol decaoleate ...... 10% Peanut oil .................... 45% Borax....................... 0. Water ..................... 37.8%
Formula//t (Mineral Oil Emulsion) Mineral oil ................... 25% Petrolatum ........... 25% Decaglycerol decaoleate ......... Decaglycerol decalinoleate ....... 3% Beeswax ....................... 7% Glycerol ...................... 3% Borax ....................... 0.5% Water ...................... 32. Formula//6 (Cosmetic Emulsion) Neobee M5 :................... 3% Cetyl alcohol ................. Decaglyceroldecalinoleate ....... Decaglyce ol monopalmitate ..... 4% Drewmulse 1128{} ............... 8%
Formula//3 (Mineral Oil Emulsion) Mineral oil (50--60risc.) ........ 38% Petrolatum ................... Decaglycerol decastearate ........ 3% Decaglycerol tetraoleate ......... 5% Lanolin ........................ Beeswax ....................... 3% Magnesiumstearate ............. Water ...................... 32.6% Borax...................... 0.4%
Formula//5 (Cosmetic Emulsion)
Isopropyl myristate .............
.4/0 Cetyl alcohol .................. Decaglycerol tetraoleate......... l Decaglycerol mono61eate ......... Stearic acid .................. 18% Spermaceti ..................... Glycerol ....................... 4% Hexachlorophene ............. 0.5% Water ...................... 66.5%
Cabosil[[ .....................
Glycerol....................... Water.......................
1%
3% 73%
* Glyceryl tripalmitate (Drew Chem. Corp.). t Modifiedcoconut triglyceride (Drew Chem.Corp.). :[: Modifiedcoconuttriglyceride (Drew Chem.Corp.). {} Self-emulsifying glycerylmonostearate (Drew Chem Corp.). [[ A gradeof silicamanufactured by G. L. CabotCo.
'I-ABLE V---ZONES OF INHIBITIOq
E. coli
, Emulsifier
POE Sorbitan
Ps. aeroginosa
Concentration of Emulsifier Used
d. niger
0 o. 5% 1.0% 2.0% 0 o. 5% 1.0% 2.0% 0 0.5% 1.0% 2.0%
7
..
mono61eate
POE Sorbitan monolaurate
5
..
8
.. -'
POE (39) stearate .. POE (23) laurylalcohol .. Decaglycerol
mono61eate* ..
10
12
10
.. ..
..
.. ..
..
Decaglycerol
monolaurate* .. 15 15 10 .. 5 3 2 .. 6 8 7
Decaglycerol monopalmitate* Hexaglycerol
monoiJleate*
..
..
12
20
8
10
10 ..
5 ..
5
5
2
1
1
-
..
..
6
9
6
7
7
3
All testswere run using0.5% G-11 in alcoholic soluiion,with and without emulsifiers.
- indicates no inhibition. Numbers indicate the zone of inhibition in ram.
* Please note that no precipitate occurred in alcohol at these concentrations of emulsifier.
POLYGLYCEROL
ESTERS
IN
PHARMACEUTICAL
PREPARATIONS
483
self-emulsifying waxes. Thus, the addition of hexaglycerol mono61eate to mineraloil produces a clearoil whichis readilyrinsedoff the handswith
water. Other esters,such as decaglyceroltetraoleate, can be used in a similarfashion with vegetable oil.
The fact that the polyglycerol estersare edibleopens up many possible applications in the pharmaceutical field. Thus, their useas emulsifiers in edibleemulsions containing vegetable oils and as solubilizers for vitamins iscurrentlyunderinvestigation.
SUMMARY
The chemical andphysical characteristics of a series of polyglycerol esters are presented. The utility of this versatilegroupof compounds is illustrated. The stability of O/W and W/O emulsions preparedwith the aid of these estersis examined. Formulationsof practical cosmetics containingpolyglycerol esters are given. Preliminarytestssuggest that these emulsifiers do not interferewith the activity of Hexachlorophene.
(ReceivedJanuary 15, 1964)
REFERENCES
(1) W. C. Griffinin E. Sagatin,Cosmetics: Science and Technology, Interscience Publishers, Inc., New York, 1004-05 (1955). (2) W. F. Rehm,Milchwissenschaft, 16, 310 (1961). (3) A. Bille and A. Mirimanoff, y. Pharm. Pharmacol., 2, 685 (1950). (4) M. G. deNavarreandH. E),Bailey,y.Soc.Cosmetic Chemists, 7, 427 (1956). (5) M. G. deNavarre,Ibid., 8, 68 (1957). (6) L. F. Tice and M. Bart, Ibid., 9, 171 (1958). (7) D. L. Wedderburn, Ibid., 9, 210 (1958). (8) R.G. Harry, ModernCosmeticology, LeonardHill (Books)Ltd., London,1962,p. 566. (9) N.H. Nash and V. K. Babayan,Baker'sDigest,37, 72 (1963). (10) Patent PendingSerialNo. 14477. (11) K. S. Markley, Fatty .4cids, Part 2, Interscience Publishers, Inc., New York, 1961,pp.
826-27.
(12) H. Kaunits,C. A. SlanetzandV. K. Babayan,y..4m. 01l Chemists' Soc.,41, 434 (1964). (13) Federal Register, July 2, 1963,p. 6783; March 19, 1963,pp. 2675-76.
(15) W. C. Griffinin E. Sagatin, Cosmetics: Science and Technology, Interscience Publishers, Inc., New York, 1955,.pp.1018-20. (16) [dem., Ibid., p. 1029.
(14) W. C. Griffin, y. Soc. Cosmetic Chemists, 1, 311(1949).
You might also like
- Safety Assessment of Polyglyceryl Fatty Acid Esters As Used in CosmeticsDocument99 pagesSafety Assessment of Polyglyceryl Fatty Acid Esters As Used in CosmeticsMEL ESPINOZA RENGIFONo ratings yet
- Formal Report FamsDocument4 pagesFormal Report FamsAlexander David FamadulanNo ratings yet
- GlycerolDocument18 pagesGlycerolQuyen Luong100% (1)
- Pharmaceutical SciencesDocument9 pagesPharmaceutical SciencesJames HornerNo ratings yet
- (ARTICULO) Caracterizacion de Lipasa Mucor Racemosus Con Aplicacion Potencial en El Tratamiento de La CelulitisDocument7 pages(ARTICULO) Caracterizacion de Lipasa Mucor Racemosus Con Aplicacion Potencial en El Tratamiento de La CelulitisAle EstradaNo ratings yet
- Lipids Act4Document21 pagesLipids Act4Nikko Mabbayad50% (2)
- Target: LipidsDocument12 pagesTarget: LipidsFeaid Aina OrnedoNo ratings yet
- Research and Reviews: Journal of Pharmacy and Pharmaceutical SciencesDocument11 pagesResearch and Reviews: Journal of Pharmacy and Pharmaceutical SciencesSurendar KesavanNo ratings yet
- UNIT4 Expt1Document6 pagesUNIT4 Expt1Christian Franco RuizNo ratings yet
- Biochem 34 FR Expt #7 & 9Document10 pagesBiochem 34 FR Expt #7 & 9louize_1496No ratings yet
- Isolation and Characterization of CarbohydratesDocument4 pagesIsolation and Characterization of CarbohydratesEvans DionNo ratings yet
- CellulosDocument14 pagesCellulosBasava PrasadNo ratings yet
- Auto Associative AmphiphilicDocument7 pagesAuto Associative AmphiphilicValentina RoznovNo ratings yet
- Isolation, General Tests, and Hydrolysis of Polysaccharides Formal ReportDocument4 pagesIsolation, General Tests, and Hydrolysis of Polysaccharides Formal ReportJuliefer May Fanilag Pleños100% (1)
- Colloids and Surfaces B: BiointerfacesDocument10 pagesColloids and Surfaces B: BiointerfaceszulhairiNo ratings yet
- Molecules 23 00357Document10 pagesMolecules 23 00357Jorge RuizNo ratings yet
- Drug DeliveryDocument11 pagesDrug DeliveryKamlesh raiNo ratings yet
- Analysis of Mixtures Containing Free Fatty Acids and Mono-, Di - and Triglycerides by High-Performance Liquid Chromatography Coupled With Evaporative Light-Scattering DetectionDocument8 pagesAnalysis of Mixtures Containing Free Fatty Acids and Mono-, Di - and Triglycerides by High-Performance Liquid Chromatography Coupled With Evaporative Light-Scattering Detectionpcdupuis8828No ratings yet
- Extraction and Hydrolysis of Glycogen and Characterization Tests For CarbohydratesDocument7 pagesExtraction and Hydrolysis of Glycogen and Characterization Tests For CarbohydratesangelsiopaoNo ratings yet
- Experiment 5a Pre Post LabDocument9 pagesExperiment 5a Pre Post LabRue Cheng Ma100% (1)
- Glucose Sensitive HydrogelsDocument4 pagesGlucose Sensitive HydrogelsJanviNo ratings yet
- LipidDocument49 pagesLipidLe Thanh HaiNo ratings yet
- OF CVPsDocument18 pagesOF CVPsClassic AddaNo ratings yet
- Car BomerDocument8 pagesCar BomerEr RicitosNo ratings yet
- Acid Value of OilDocument20 pagesAcid Value of OilFavourNo ratings yet
- 2 Biological MoleculesDocument82 pages2 Biological MoleculesgyunimNo ratings yet
- MC2 Biochemistry Lecture Notes For BSN First Semester, 2019-2020 Prepared By: SALINA OSIAL - ALFADDocument5 pagesMC2 Biochemistry Lecture Notes For BSN First Semester, 2019-2020 Prepared By: SALINA OSIAL - ALFADAl-waleed JulkanainNo ratings yet
- Experiment 4 LipidsDocument11 pagesExperiment 4 Lipidsiey ranaNo ratings yet
- HPLC For Carbohydrate Analysis: October 2014Document21 pagesHPLC For Carbohydrate Analysis: October 2014Julia Zahra ArdiantiNo ratings yet
- Figure 1. Structure of PLGADocument5 pagesFigure 1. Structure of PLGAJessie ChuNo ratings yet
- Carbo PolDocument8 pagesCarbo PolRia MardianaNo ratings yet
- CelluloseDocument7 pagesCelluloseSMIT CHRISTIANNo ratings yet
- Biology FSC Chapter 2Document15 pagesBiology FSC Chapter 2Diana PriyaNo ratings yet
- Characterization of Nata de Coco Produced by Fermentation of Immobilized Acetobacter XylinumDocument8 pagesCharacterization of Nata de Coco Produced by Fermentation of Immobilized Acetobacter XylinumJay MeeNo ratings yet
- Glycolic AcidDocument7 pagesGlycolic AcidJoseph MedinaNo ratings yet
- Carbopol Polymers Are Offered As Fluffy, White, Dry Powders (100% Effective)Document12 pagesCarbopol Polymers Are Offered As Fluffy, White, Dry Powders (100% Effective)akromulr100% (1)
- AquaSolve As HandbookDocument16 pagesAquaSolve As Handbookmaneshdixit4312No ratings yet
- Unit 3&4 Exercises Biochemistry 17.11.20Document36 pagesUnit 3&4 Exercises Biochemistry 17.11.20Nguyen Bao TranNo ratings yet
- BiomoleculesDocument10 pagesBiomoleculeststylizeNo ratings yet
- Qualitativetestsforcarbohydrates 140615032421 Phpapp01Document52 pagesQualitativetestsforcarbohydrates 140615032421 Phpapp01arun231187No ratings yet
- Chemistry Notes For Class 12 Chapter 14 BiomoleculesDocument13 pagesChemistry Notes For Class 12 Chapter 14 Biomoleculesrathi rupaNo ratings yet
- 08LipAMO SuarezDocument15 pages08LipAMO SuarezscasuarezNo ratings yet
- BiochemistryDocument6 pagesBiochemistrySofia AbainciaNo ratings yet
- Starch Based Polymer PDFDocument10 pagesStarch Based Polymer PDFSachikanta PradhanNo ratings yet
- Test For Sugars PDFDocument12 pagesTest For Sugars PDFRut ChristineNo ratings yet
- Analysis of LipidsDocument11 pagesAnalysis of LipidsSundaram PattaruNo ratings yet
- Music Acid TestDocument3 pagesMusic Acid TestainakmliaNo ratings yet
- Applsci 11 05152Document17 pagesApplsci 11 05152siti fajar karinaNo ratings yet
- Experiment 3 - CarbohydratesDocument15 pagesExperiment 3 - CarbohydratesNur Setsu100% (1)
- Importance of Cyclodextrins in Human MedicineDocument5 pagesImportance of Cyclodextrins in Human MedicineS Bharadwaj ReddyNo ratings yet
- Synthesis and Characterization of A New Cellulose Acetate-Propionate Gel: Crosslinking Density DeterminationDocument8 pagesSynthesis and Characterization of A New Cellulose Acetate-Propionate Gel: Crosslinking Density DeterminationAyus DiningsihNo ratings yet
- 5.lipid Dan Asam LemakDocument29 pages5.lipid Dan Asam LemakLatoya BaileyNo ratings yet
- LipidsDocument4 pagesLipidsStephanie Joy EscalaNo ratings yet
- Catalytic Etherification of Glycerol With AlcoholsDocument7 pagesCatalytic Etherification of Glycerol With AlcoholsPopescu IoanaNo ratings yet
- 2012 NullDocument10 pages2012 NullRagabAbdoNo ratings yet
- 5 Natural PolymersDocument10 pages5 Natural PolymersakeemNo ratings yet
- (H2) CI1.2 - Biomolecules (Lipids)Document14 pages(H2) CI1.2 - Biomolecules (Lipids)Timothy HandokoNo ratings yet
- Handbook of Food Science and Technology 1: Food Alteration and Food QualityFrom EverandHandbook of Food Science and Technology 1: Food Alteration and Food QualityNo ratings yet
- Benzoylation of AcetophenoneDocument3 pagesBenzoylation of AcetophenonetechkasambaNo ratings yet
- Sheet1 F2:F51 Contains 1 Sheet1 G2:G51 Contains 2 Sheet1 F2:F51 Contains 1 Sheet1 G2:G51 Contains 2Document9 pagesSheet1 F2:F51 Contains 1 Sheet1 G2:G51 Contains 2 Sheet1 F2:F51 Contains 1 Sheet1 G2:G51 Contains 2techkasambaNo ratings yet
- Triazoles ChemistryDocument4 pagesTriazoles ChemistrytechkasambaNo ratings yet
- S.No Company Name Price On 7 April 201 Buy Amount Quantity Current Priceprofit/LossDocument3 pagesS.No Company Name Price On 7 April 201 Buy Amount Quantity Current Priceprofit/LosstechkasambaNo ratings yet
- The Decomposition of Aqueous Sodium Bromite: Department of Chemistry, University of Toronto, Toronto OntarioDocument6 pagesThe Decomposition of Aqueous Sodium Bromite: Department of Chemistry, University of Toronto, Toronto OntariotechkasambaNo ratings yet
- Assessing The Profitability of Intraday Opening Range Breakout StrategiesDocument11 pagesAssessing The Profitability of Intraday Opening Range Breakout StrategiestechkasambaNo ratings yet
- Save Water, Save Life PDFDocument30 pagesSave Water, Save Life PDFtechkasambaNo ratings yet
- Synthesis of 2-Picoline From Acetone Over Modified ZSM-5 CatalystsDocument8 pagesSynthesis of 2-Picoline From Acetone Over Modified ZSM-5 CatalyststechkasambaNo ratings yet
- Isoamyl SalysilateDocument1 pageIsoamyl SalysilatetechkasambaNo ratings yet
- IPTFADocument9 pagesIPTFAtechkasambaNo ratings yet
- 5 Quiz 5 WifeDocument2 pages5 Quiz 5 WifetechkasambaNo ratings yet
- Stop Worrying About Making Right DecisionsDocument2 pagesStop Worrying About Making Right DecisionstechkasambaNo ratings yet
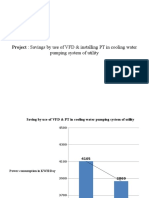
- Project: Savings by Use of VFD & Installing PT in Cooling WaterDocument6 pagesProject: Savings by Use of VFD & Installing PT in Cooling WatertechkasambaNo ratings yet
- Proposed Project: Saving in Power Consumption: by Installing VFD in N CompressorDocument3 pagesProposed Project: Saving in Power Consumption: by Installing VFD in N CompressortechkasambaNo ratings yet
- Leather and Leather Products: Food IndustriesDocument4 pagesLeather and Leather Products: Food IndustriestechkasambaNo ratings yet
- Vishranti Greens BrochureDocument14 pagesVishranti Greens BrochuretechkasambaNo ratings yet
- Lagnas CharacterDocument14 pagesLagnas CharactertechkasambaNo ratings yet
- 12 Week Bikini Bombshell Workout Plan PDFDocument9 pages12 Week Bikini Bombshell Workout Plan PDFGHOST191491100% (1)
- PQRS Practice QuestionsDocument13 pagesPQRS Practice QuestionsLucky VermaNo ratings yet
- Thesis About Monosodium GlutamateDocument33 pagesThesis About Monosodium GlutamateArrianne Jaye Mata40% (10)
- 100 Push Ups 50 Pull UpsDocument5 pages100 Push Ups 50 Pull UpsJavier AsensioNo ratings yet
- Nclex Cardio QuestionsDocument4 pagesNclex Cardio QuestionsMichelleNo ratings yet
- A Systematic Review of Dietary Protein During Caloric Restriction in Resistance Trained Lean Athletes: A Case For Higher IntakesDocument13 pagesA Systematic Review of Dietary Protein During Caloric Restriction in Resistance Trained Lean Athletes: A Case For Higher IntakesGeorge BrianNo ratings yet
- The Impact of Social Media On The Body - Selma DajaniDocument14 pagesThe Impact of Social Media On The Body - Selma Dajaniapi-286617186No ratings yet
- Core Fitness Zone Resistance BandsDocument47 pagesCore Fitness Zone Resistance BandsSandesh100% (5)
- Antidiabetic Activity of Chandraprabha Vati e A Classical AyurvedicDocument7 pagesAntidiabetic Activity of Chandraprabha Vati e A Classical AyurvedicChaitanya M MundheNo ratings yet
- Health Teaching PlanDocument10 pagesHealth Teaching PlanMariel Colminas100% (2)
- Puberty and The HPG AxisDocument36 pagesPuberty and The HPG AxiskjhkaNo ratings yet
- Soto 9780307594884 1p All r1Document336 pagesSoto 9780307594884 1p All r1wamu8850No ratings yet
- Initial Management of Blood Glucose in Adults With Type 2 Diabetes Mellitus - UpToDateDocument24 pagesInitial Management of Blood Glucose in Adults With Type 2 Diabetes Mellitus - UpToDateJessica ArciniegasNo ratings yet
- Energy Requirements For AdultsDocument3 pagesEnergy Requirements For AdultsSyifa MustikaNo ratings yet
- 7.1 7.2 Animal Nutrition Alimentary CanalDocument6 pages7.1 7.2 Animal Nutrition Alimentary CanalgesNo ratings yet
- Argumentative EssayDocument6 pagesArgumentative Essayapi-331322127No ratings yet
- 8 LipoproteinsDocument12 pages8 LipoproteinsSubhi Mishra100% (1)
- ABG Interpretation: Julie Perkins RRT-NPS LPCH Respiratory CareDocument14 pagesABG Interpretation: Julie Perkins RRT-NPS LPCH Respiratory CareYS NateNo ratings yet
- Sugar Addiction - Sugar DetoxDocument30 pagesSugar Addiction - Sugar DetoxBrian StaceyNo ratings yet
- Ijem 14 04 36727Document11 pagesIjem 14 04 36727brahim azoulayeNo ratings yet
- Periodization PowerDocument3 pagesPeriodization Powertinerete_100% (1)
- Journal of Internal Medicine - 2022 - Paternostro - Current Treatment of Non Alcoholic Fatty Liver DiseaseDocument15 pagesJournal of Internal Medicine - 2022 - Paternostro - Current Treatment of Non Alcoholic Fatty Liver DiseaseHunny BunnyNo ratings yet
- Nutritional Disorders 1Document55 pagesNutritional Disorders 1nukenNo ratings yet
- Analysis: Dietary and Nutritional Approaches For Prevention and Management of Type 2 DiabetesDocument9 pagesAnalysis: Dietary and Nutritional Approaches For Prevention and Management of Type 2 DiabetesMr. LNo ratings yet
- Imagen Corporal CulturaDocument4 pagesImagen Corporal CulturaLady SanchezNo ratings yet
- Muscle Maximum Growth IIDocument116 pagesMuscle Maximum Growth IIAlex Antoniou88% (16)
- WhatsApp Chat With Sridevi RammohanDocument4 pagesWhatsApp Chat With Sridevi RammohannrcagroNo ratings yet
- Cesarean Delivery of The Obese Woman - UpToDateDocument25 pagesCesarean Delivery of The Obese Woman - UpToDateBárbara JunqueiraNo ratings yet
- Kim (2015) - Lemon Detox Diet Reduced Body FatDocument12 pagesKim (2015) - Lemon Detox Diet Reduced Body FatRodrigo MelloNo ratings yet
- Histologi KardiovaskularDocument32 pagesHistologi KardiovaskularMochammad Fariz AmsalNo ratings yet