Professional Documents
Culture Documents
Ards PDF
Uploaded by
Angela PagliusoOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Ards PDF
Uploaded by
Angela PagliusoCopyright:
Available Formats
ARDS: Case Study of a Mystery Killer
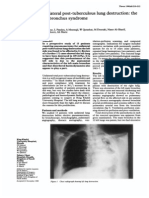
Dr. C. Chan Gunn Order of British Columbia MD, PhD (hon.), DSc (hon.) Adult Respiratory Distress Syndrome (ARDS) is a dangerous lung condition brought on by various diseases and injuries. It is an acute failure of the respiratory system distinguished by fluid buildup inside the lungs, causing stiffening and decreased lung capacity. Symptoms include damage to endothelium and lining, low O2 concentration in blood despite heavy oxygen supplements given to the patient, inflammation of lung cells, and loss of consciousness.1 ARDS kills more than 60% of its victims. Over 100,000 people in the United States die from ARDS each year -- a higher death rate than AIDS.2 In the 25 years since it was first described, few advances have been made to reduce the death rate of ARDS. To devise effective treatments, we need a better understanding of the physiological process responsible for the syndrome. Ware and Matthay described in their recent paper3 some of the advancements in the understanding of ARDS, but cited little progress in its specific treatment. Perhaps we lack successful treatment because we have not recognized that the pleural effusion in ARDS involves neuroinflammation and gap formation. I had the unfortunate opportunity for a case study on this aspect of ARDS when it struck my wife (68 and previously healthy). She was admitted to ICU on December 28, 1999 with acute respiratory failure secondary to viral pneumonia. Her condition deteriorated rapidly and she required intubation and mechanical ventilation: she was unconscious for 30 days. Her x-ray reports document her progress. 12/28/99moderate right upper lobe collapse and consolidation...air space disease involving the left lower lobeill-defined and nodular opacities in the left mid lungheart not enlargedno evidence of mediastinal or hilar adenopathy. 12/29/99 - Lung volumes have diminishedworsening and more confluent opacification of the left midlung. In addition, new airspace disease in the right middle lobe. The previously described regions of collapse and consolidation in the right upper and left lower lobes have become denser as a result of diminished lung volumes. Overall worsening. 12/29/99 - Patient has been intubated There is worsening air space disease involving both lungs, which appears worse even though the lung volumes have improved as the result of the intubation. Although distribution would seem in keeping with an infectious etiology, the rapidity of the changes raises the possibility of superimposed non-cardiogenic pulmonary edema. She required tracheostomy and pure oxygen was administered at maximum pressure. She developed atrial fibrillation which required cardioversion and Type II diabetes. When X-rays on 1/2/00 showed no improvement, the attending physician sadly informed our family that her condition was irreversible; she was kept alive only by ICU support. I asked permission to examine her and recognized her physical signs to be those of radiculopathy involving several segments.4 For example: atrophy of the small muscles in her hands (C7-8); severe atrophy of the gluteal muscles and decubitus ulcers in the buttocks and left heel (L5, S1-2); trophedema in the subcutaneous tissues of her dorsal and lumbar backs. Lungs are innervated by nerves from lower cervical and upper dorsal segments.
The pulmonary edema was likely part of the neuropathic disorder. In desperation, I called upon low level laser therapy, a treatment that is used by physical therapists to soothe inflammation and dissipate edema in soft tissue injury. I directed the laser beam (820nm and 50 mw) between her ribs, into the lungs, and also into spinal muscles overlying affected nerve roots. X-rays taken 6 hours later showed a change for the better: 1/3/00 - The consolidative changes in the left upper lobe are slightly reduced. The right upper lobe appears slightly better aerated. The right lower lobe also shows some decrease in the amount of consolidation. Impression: The patient with known diffuse bilateral pneumonia shows slight improvement in the left upper lobe and right lower lobe. 1/7/00 - Both lungs are well ventilated. There is widespread interstitial disease in both lungs. In the left base it has taken a honeycomb pattern. There is no lung contracture. 1/15/00 - Both lungs are well ventilated. The interstitial thickening is now localized to the periphery of the right upper zone, right lower zone and left lower zone. 1/20/00 - Patchy interstitial thickening in the periphery of both lungs. No consolidation or pulmonary edema. From commencement of daily laser therapy, it had taken 17 days to clear the lungs. John Hunter in 1794 had recognized that inflammation is a more or less stereotyped response to injury. Therefore similarities can be expected between inflammation in soft tissue and in lung and review of one may provide clues to the nature of the other. Inflammation in soft tissue causes the permeability of small blood vessels in and around the injured area to increase. This allows large amounts of fluid and plasma protein to escape into the extravascular space to form inflammatory exudate. Inflammatory exudate is not a static puddle but a high-turnover pool with a massive amount of protein passing from blood to lymph.5 While small molecules rapidly diffuse across the vascular wall, plasma protein escapes through transient gaps that appear in the junctions between adjacent endothelial cells. Gap formation, which is initiated by histamine and like substances, is believed to be caused by activation of a contractile protein, similar to actomyosin, within the cytoplasm of endothelial cells. Gap formation is a reversible process: when leakage ceases, the cells come together again without any evidence of damage to endohelial cells.6 Since inflammation in the lung is likewise associated with loss of epithelial integrity and influx of protein-rich edema fluid, gap formation likely also occurs in the lung.
Soft tissue inflammation generally follows injury, but it can (like soft tissue pain) also result from peripheral neuropathy. Radiculopathy is not uncommon in the very young and old, the two age groups that are at risk. Is lung inflammation, generally associated with injury, a neuropathic manifestation? These are questions that may be answered by searching for signs of peripheral neuropathy in patients with respiratory distress. But, whether or not the condition is related to neuropathy, low level laser therapy, a simple modality with few undesirable side-effects should be tried. The decubitus ulcers, well known for their reluctance to heal, were cleared in days. Myotomal muscle wasting was electrically stimulated with a transcutaneous electrical stimulator [TENS]. Treatment should preferably be early in the course of the disease.
References Greene A et al. ARDS (adult respiratory distress syndrome). Health Illustrated Encyclopedia. A.D.A.M. 2000. Repine J. Adult Respiratory Distress Syndrome (ARDS): The mystery killer. Webb-Waring Institute. 2000 http://www.uchsc.edu/sm/waring/webpages/secondlevel/science/ards.html Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000; 342 18 1334-49. Gunn CC. Early and subtle signs in low-back sprain. Spine 1978, 3 3 100- 114. Pontinen PJ. Low Laser Therapy As A Medical Treatment Modality. Art Upo, Tampere 1992. Hurley JV. In EDEMA, Editors, Staub and Taylor, Raven Press, New York, 1984; 463-488.
You might also like
- Acute Respiratory Distress SyndromeDocument21 pagesAcute Respiratory Distress Syndromemisseve252100% (1)
- Description of DiseaseDocument10 pagesDescription of DiseaseRajaNo ratings yet
- Adult Respiratory Distress Syndrome: ReviewDocument5 pagesAdult Respiratory Distress Syndrome: ReviewJuliana Rodriguez MariñoNo ratings yet
- An Unlikely Cause: What Led To This Drug User's Shortness of Breath?Document2 pagesAn Unlikely Cause: What Led To This Drug User's Shortness of Breath?Ladislau L. CorneliussNo ratings yet
- ArdsDocument53 pagesArdsSophy Sony100% (3)
- Manajemen VentiDocument2 pagesManajemen VentiBos AseNo ratings yet
- Geriatric Case StudyDocument15 pagesGeriatric Case StudyJobelle AcenaNo ratings yet
- Cor Pulmonale An Overview 2003Document12 pagesCor Pulmonale An Overview 2003Ade Cahyo IslamiNo ratings yet
- Acute Respiratory Distress Syndrome - Background, Pathophysiology, EtiologyDocument5 pagesAcute Respiratory Distress Syndrome - Background, Pathophysiology, EtiologyARHNo ratings yet
- Ards 2Document19 pagesArds 2Augusto ManoelNo ratings yet
- Symposium Papers: What Is The Acute Respiratory Distress Syndrome?Document7 pagesSymposium Papers: What Is The Acute Respiratory Distress Syndrome?Zulkifli SalimNo ratings yet
- Acute Respiratory Distress Syndrome: David SweetDocument204 pagesAcute Respiratory Distress Syndrome: David SweetJelenaNo ratings yet
- 1 s2.0 S2213007121000289 MainDocument4 pages1 s2.0 S2213007121000289 MainHi GamingNo ratings yet
- Pulmonary Tuberculosis Presenting With Acute Respiratory Distress Syndrome (Ards) : A Case Report and Review of LiteratureDocument5 pagesPulmonary Tuberculosis Presenting With Acute Respiratory Distress Syndrome (Ards) : A Case Report and Review of Literatureamelya asryNo ratings yet
- Bilateral Massive Pulmonary Embolism Secondary To Decompression Sickness: A Case ReportDocument4 pagesBilateral Massive Pulmonary Embolism Secondary To Decompression Sickness: A Case ReportPatricio Toledo FuenzalidaNo ratings yet
- Acute Pulmonary OdemaDocument9 pagesAcute Pulmonary OdemaAnonymous ysrxggk21cNo ratings yet
- Dr. Rajmani: Assistant Professor Department of Medicine J.L.N. Medical College, Ajmer (Raj.)Document63 pagesDr. Rajmani: Assistant Professor Department of Medicine J.L.N. Medical College, Ajmer (Raj.)Ashish SoniNo ratings yet
- Acute Respiratory Distress Syndrome: Jesse B. Hall, MD, FCCPDocument12 pagesAcute Respiratory Distress Syndrome: Jesse B. Hall, MD, FCCPMayun CacoellNo ratings yet
- Acute Respiratory Distress Syndrome - Epidemiology, Pathophysiology, Pathology, and Etiology in Adults - UpToDateDocument32 pagesAcute Respiratory Distress Syndrome - Epidemiology, Pathophysiology, Pathology, and Etiology in Adults - UpToDatecuentaparatrabajosdelau10No ratings yet
- 6 - WolfeDocument25 pages6 - WolfeEzeBorjesNo ratings yet
- Infected Pulmonary Infarction Case ReportDocument8 pagesInfected Pulmonary Infarction Case ReportSebastian Felipe Sierra UmañaNo ratings yet
- Tension PneumothoraxDocument40 pagesTension Pneumothoraxbemi lestariNo ratings yet
- PneumoniaDocument24 pagesPneumoniaMuhammad HassanNo ratings yet
- Acute Lung InjuryDocument18 pagesAcute Lung InjuryM Rizal IsburhanNo ratings yet
- Emphysema Is A LongDocument54 pagesEmphysema Is A LongryemoralesNo ratings yet
- PDF To WordDocument17 pagesPDF To Wordanisa ghinaNo ratings yet
- Pulmonology MCQsDocument49 pagesPulmonology MCQsaliakbar178100% (1)
- Acute Respiratory Distress SyndromeDocument9 pagesAcute Respiratory Distress SyndromeMatthew Ryan100% (2)
- Letter TO THE Editor: Secondary Organizing Pneumonia After Varicella-Zoster Virus Infection: A Rare AssociationDocument3 pagesLetter TO THE Editor: Secondary Organizing Pneumonia After Varicella-Zoster Virus Infection: A Rare AssociationfatimiahmastaNo ratings yet
- Approach To Adult Patients With Acute DyspneaDocument21 pagesApproach To Adult Patients With Acute DyspneaCándida DíazNo ratings yet
- Interstitial Lung Diseases Radiology 22222Document26 pagesInterstitial Lung Diseases Radiology 22222Daniel AshooriNo ratings yet
- 12 RLDDocument43 pages12 RLDNur akilaNo ratings yet
- It 25 - JHP RLDDocument52 pagesIt 25 - JHP RLDkalajengkingkalakalaNo ratings yet
- Interstitial Lung Diseases RadiologyDocument26 pagesInterstitial Lung Diseases RadiologyDaniel AshooriNo ratings yet
- Ards 2Document7 pagesArds 2LUCIBELLOT1No ratings yet
- Early Diagnosis of Kartagener's SyndromeDocument8 pagesEarly Diagnosis of Kartagener's SyndromeHendra SusantoNo ratings yet
- Superior Vena Cava Syndrome Case FileDocument2 pagesSuperior Vena Cava Syndrome Case Filehttps://medical-phd.blogspot.comNo ratings yet
- PulmonologyDocument175 pagesPulmonologyJohanna GarciaNo ratings yet
- Acute Respiratory Distress Syndrome....Document55 pagesAcute Respiratory Distress Syndrome....sangi vinayagamNo ratings yet
- Case Presentation MucormycosisDocument10 pagesCase Presentation MucormycosiskunaidongNo ratings yet
- Low-Tidal-Volume Ventilation in The Acute Respiratory DistressDocument12 pagesLow-Tidal-Volume Ventilation in The Acute Respiratory DistressJesus FrancoNo ratings yet
- Low-Tidal-Volume Ventilation in The Acute Respiratory Distress SyndromeDocument8 pagesLow-Tidal-Volume Ventilation in The Acute Respiratory Distress SyndromeJimmy Christianto SuryoNo ratings yet
- Respiratory diseases guideDocument71 pagesRespiratory diseases guideZafir SharifNo ratings yet
- Acute Lung InjuryDocument5 pagesAcute Lung InjuryKinanti Devia LarasatiNo ratings yet
- A 56 Year Old Man With Chronic Cough, HemoptysisDocument4 pagesA 56 Year Old Man With Chronic Cough, HemoptysisagamerocallejasNo ratings yet
- Journal Radiologi 3Document14 pagesJournal Radiologi 3WinayNayNo ratings yet
- Pneumonitis Ec ChemotheraphyDocument5 pagesPneumonitis Ec ChemotheraphyNurafiat AntonNo ratings yet
- Respiration CH 43.Dr SarahDocument59 pagesRespiration CH 43.Dr Sarahaiman siddiquiNo ratings yet
- Sweier Jamrs SyndromDocument4 pagesSweier Jamrs SyndromBulborea MihaelaNo ratings yet
- Adult Respiratory EmergenciesDocument39 pagesAdult Respiratory EmergenciesKofi DanielNo ratings yet
- ARDS EngDocument25 pagesARDS EngTofik ShehajNo ratings yet
- Emphysema Chronic Obstructive Pulmonary Disease (COPD) : Call Dasco Today For More Information 855-442-7912Document6 pagesEmphysema Chronic Obstructive Pulmonary Disease (COPD) : Call Dasco Today For More Information 855-442-7912Nazif Aiman IsmailNo ratings yet
- Noncardiogenic Pulmonary Edema in Small AnimalsDocument17 pagesNoncardiogenic Pulmonary Edema in Small AnimalsKaren PMNo ratings yet
- PDF JournalDocument5 pagesPDF JournalTina de JesusNo ratings yet
- Acute Respiratory Distress SyndromeDocument17 pagesAcute Respiratory Distress SyndromeSanjeet SahNo ratings yet
- CHP 11 Cerebral Air EmbolismDocument8 pagesCHP 11 Cerebral Air EmbolismSrinivas GokulnathNo ratings yet
- Interstitial Lung DiseaseDocument14 pagesInterstitial Lung DiseaseAzkaZulfiqarNo ratings yet
- Left Bronchus SyndromeDocument3 pagesLeft Bronchus SyndromephobicmdNo ratings yet
- The Unified Airway: Rhinologic Disease and Respiratory DisordersFrom EverandThe Unified Airway: Rhinologic Disease and Respiratory DisordersDavid A. GudisNo ratings yet
- Medicine in Brief: Name the Disease in Haiku, Tanka and ArtFrom EverandMedicine in Brief: Name the Disease in Haiku, Tanka and ArtRating: 5 out of 5 stars5/5 (1)
- Peripheral Nerve Entrapment Hydrodissection and Neural Regenerative Strategies Andrea Trescot 2015 PDFDocument9 pagesPeripheral Nerve Entrapment Hydrodissection and Neural Regenerative Strategies Andrea Trescot 2015 PDFAngela PagliusoNo ratings yet
- Intraneural Inistion EcoguidatoDocument7 pagesIntraneural Inistion EcoguidatoAngela PagliusoNo ratings yet
- PerineuralDocument5 pagesPerineuralAngela PagliusoNo ratings yet
- Peripheral Nerve Entrapment Hydrodissection and Neural Regenerative Strategies Andrea Trescot 2015 PDFDocument9 pagesPeripheral Nerve Entrapment Hydrodissection and Neural Regenerative Strategies Andrea Trescot 2015 PDFAngela PagliusoNo ratings yet
- Embedding AcupunctureDocument5 pagesEmbedding AcupunctureAngela PagliusoNo ratings yet
- Washington State PNT Study Finds Specific Needle Placement Most EffectiveDocument9 pagesWashington State PNT Study Finds Specific Needle Placement Most EffectiveAngela PagliusoNo ratings yet
- C MaxwellDocument4 pagesC MaxwellAngela PagliusoNo ratings yet
- Agopuntura Per StrokeDocument14 pagesAgopuntura Per StrokeAngela PagliusoNo ratings yet
- Acupuncture Obesity PDFDocument27 pagesAcupuncture Obesity PDFRamonRamosSepúlvedaOliveiraNo ratings yet
- Agopuntura Per StrokeDocument14 pagesAgopuntura Per StrokeAngela PagliusoNo ratings yet
- Lavoro Su Agopuntura RiscaldataDocument8 pagesLavoro Su Agopuntura RiscaldataAngela PagliusoNo ratings yet
- The Acumoxa Treatment of Shoulder Impingement SyndromeDocument8 pagesThe Acumoxa Treatment of Shoulder Impingement SyndromeAngela PagliusoNo ratings yet
- Om NL Summer 2010Document11 pagesOm NL Summer 2010Angela PagliusoNo ratings yet
- Agopuntura Per StrokeDocument14 pagesAgopuntura Per StrokeAngela PagliusoNo ratings yet
- Acupuncture Obesity PDFDocument27 pagesAcupuncture Obesity PDFRamonRamosSepúlvedaOliveiraNo ratings yet
- The Acumoxa Treatment of Shoulder Impingement SyndromeDocument8 pagesThe Acumoxa Treatment of Shoulder Impingement SyndromeAngela PagliusoNo ratings yet
- Acupuncture Obesity PDFDocument27 pagesAcupuncture Obesity PDFRamonRamosSepúlvedaOliveiraNo ratings yet
- The Acumoxa Treatment of Shoulder Impingement SyndromeDocument8 pagesThe Acumoxa Treatment of Shoulder Impingement SyndromeAngela PagliusoNo ratings yet
- Washington State PNT Study Finds Specific Needle Placement Most EffectiveDocument9 pagesWashington State PNT Study Finds Specific Needle Placement Most EffectiveAngela PagliusoNo ratings yet
- Lavoro Su Agopuntura RiscaldataDocument8 pagesLavoro Su Agopuntura RiscaldataAngela PagliusoNo ratings yet
- NeuralRegenRes101128-5765707 160057Document8 pagesNeuralRegenRes101128-5765707 160057Angela Pagliuso100% (1)
- Therapeutic Approach of Acupuncture For Sciatica A Brief ReviewDocument7 pagesTherapeutic Approach of Acupuncture For Sciatica A Brief ReviewAngela PagliusoNo ratings yet
- Pelvic Pain Relief TrialDocument5 pagesPelvic Pain Relief TrialAngela PagliusoNo ratings yet
- Fire NeedlingDocument4 pagesFire NeedlingAngela PagliusoNo ratings yet
- Eacupuncture For SciaticaDocument4 pagesEacupuncture For SciaticaAngela PagliusoNo ratings yet
- 2006 ICAET SmallDocument32 pages2006 ICAET SmallAngela PagliusoNo ratings yet
- Vit C PerineuraleDocument3 pagesVit C PerineuraleAngela PagliusoNo ratings yet
- J J Rad Oncol 3 2 029-2Document4 pagesJ J Rad Oncol 3 2 029-2Angela PagliusoNo ratings yet
- Systemic Inflammation and Disease Progression in Alzheimer DiseaseDocument7 pagesSystemic Inflammation and Disease Progression in Alzheimer DiseaseAngela PagliusoNo ratings yet
- Etanercept Post Strok RecoveryDocument19 pagesEtanercept Post Strok RecoverySomu VictorNo ratings yet
- Diabetes MellitusDocument6 pagesDiabetes MellitusDr Mangesti Utami PKM Kebaman BanyuwangiNo ratings yet
- Tangled ScriptDocument5 pagesTangled ScriptMikaela Nueva100% (1)
- Obesity and MetabolismDocument305 pagesObesity and Metabolismsavvy_as_98100% (1)
- Geriatric Nursing Terms and ConceptsDocument53 pagesGeriatric Nursing Terms and ConceptsGodfrey FrancoNo ratings yet
- Assisted Suicide ViewpointsDocument221 pagesAssisted Suicide ViewpointsMuneeb ArshadNo ratings yet
- PHICDocument3 pagesPHICapi-3836762No ratings yet
- Pancreatita Acuta Curs PT Rezidenti AJRDocument7 pagesPancreatita Acuta Curs PT Rezidenti AJRpunct_org3256No ratings yet
- Sindh Nurses Examination Board KarachiDocument8 pagesSindh Nurses Examination Board KarachiBurhan uddin100% (13)
- 1 - Goljan - Cell InjuryDocument10 pages1 - Goljan - Cell Injuryjhk451No ratings yet
- MeningiomaDocument7 pagesMeningiomadrhendyjuniorNo ratings yet
- Presentación DR Nagy Budapest1 2013 (Estética)Document107 pagesPresentación DR Nagy Budapest1 2013 (Estética)Doctora Santana100% (1)
- Case Study 2 - PancreatitisDocument1 pageCase Study 2 - Pancreatitisapi-389612279No ratings yet
- Pharmacology Reviewer #01Document21 pagesPharmacology Reviewer #01Cutie Patootie100% (1)
- The Hall of Fire 06Document27 pagesThe Hall of Fire 06maldreidorNo ratings yet
- Peripheral Nerve Injuries ExplainedDocument4 pagesPeripheral Nerve Injuries ExplainedJianni ClaridadNo ratings yet
- BJD 1348Document12 pagesBJD 1348Maciej BuczNo ratings yet
- STI College of Nursing Sta. Cruz, Laguna College of Nursing: Submitted To: Mrs. Aurea Celino, RN Clinical InstructorDocument41 pagesSTI College of Nursing Sta. Cruz, Laguna College of Nursing: Submitted To: Mrs. Aurea Celino, RN Clinical InstructorPong's Teodoro SalvadorNo ratings yet
- The Bedside Oral Exam and The Barrow Oral Care ProtocolDocument2 pagesThe Bedside Oral Exam and The Barrow Oral Care ProtocolKarla Katrina CajigalNo ratings yet
- Daftar Poster CaseDocument6 pagesDaftar Poster Casebagusputrabali13No ratings yet
- Erik Ravelo 10-11 AssignmentDocument9 pagesErik Ravelo 10-11 Assignmentapi-238741224No ratings yet
- Research Institute For Tropical Medicine: Admitted in Multiple Health FacilitiesDocument5 pagesResearch Institute For Tropical Medicine: Admitted in Multiple Health FacilitiesYescha FabitoNo ratings yet
- Answer Key On Human BehaviorDocument8 pagesAnswer Key On Human BehaviorNhickoLetKwOn100% (1)
- AtilomataDocument7 pagesAtilomatamilindNo ratings yet
- DM 2 Case PresDocument132 pagesDM 2 Case Prestintin srgpnNo ratings yet
- Diabetic Foot Ulcer Internist ApproachDocument57 pagesDiabetic Foot Ulcer Internist ApproachSandip DNo ratings yet
- Bio ReportDocument4 pagesBio ReportKate MabanagNo ratings yet
- Immediate Nursing Interventions for Common Medical ConditionsDocument91 pagesImmediate Nursing Interventions for Common Medical ConditionsElizalde HusbandNo ratings yet
- Plant Protection Cardamom: Presented byDocument26 pagesPlant Protection Cardamom: Presented byaditya rajNo ratings yet
- Stress Levels of Employees in BPO IndustryDocument37 pagesStress Levels of Employees in BPO IndustryKaushik Iyer100% (2)
- Wiccan Herbs for Protection, Healing, and MoreDocument10 pagesWiccan Herbs for Protection, Healing, and MoreDyana MariaNo ratings yet