Professional Documents
Culture Documents
Benzoic
Uploaded by
dsdeCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Benzoic
Uploaded by
dsdeCopyright:
Available Formats
-1-
DISTRIBUTION OF BENZOIC ACID BETWEEN TOLUENE AND WATER AND DIMERIZATION OF BENZOIC ACID IN TOLUENE
OBJECTIVE: In this experiment we will use the volumetric technique of acid-base titration to determine the distribution of benzoic acid in the water-toluene system. From these distribution measurements we will be able to examine the two equilibria: (1) dimerization of benzoic acid in toluene and, (2) distribution of benzoic acid monomers between water and toluene. INTRODUCTION: A general treatment by Moelwyn-Hughes1 allows examination of the above two equilibria from distribution measurements. The benzoic acid water-benzene system has been studied by Huq and Lodhi2. This treatment has been extended to other acids (e.g. acetic and propionic acids) distributed between water and other organic solvents3. In the benzoic acid-water-toluene system, there are three equilibria which must be considered. In the aqueous layer, benzoic acid, being a weak acid will dissociate: HBz
k H (aq) + Bz (aq)
+ -
(1)
and we can write the acid dissociation constant, Ka for the equilibrium as
+ Ka = [H ] [Bz ] [HBz]W
(2)
In the toluene layer, benzoic acid exists both as a monomer and dimer (i.e. two benzoic acid molecules bonded together by hydrogen bonding to form a new species). This dimerization is also an equilibrium: (3) 2 HBz (HBz)2
KD =
[(HBz)2]T [HBz]T
2
(4)
where [HBz]T, is the equilibrium molar concentration of the monomer in toluene and [(HBz)2]T is the equilibrium molar concentration of the dimer in toluene. The third equilibrium is the equilibrium constant for the distribution of benzoic acid monomers between water and benzene: (HBz)W KM =
[HBz]T
k (HBz)
(5) (6)
[HBz]W
-2The total concentrations of benzoic acid in water and toluene, CW and CT respectively, is obtained by titration of the appropriate phase with sodium hydroxide. CW = [HBz]W + [Bz-]W = CW(1 - ) + CW where is the degree of dissociation of benzoic acid; and CT = [HBz]T + 2 [(HBz)2]T (8) (7)
Using Eqs.(6) and (4) as well as noting that [HBz]W = CW(1 - ) allows Eq.(8) to be rearranged to, CT CW(1 - )
= KM + 2KMKDCW(1 - )
(9)
Eq.(9) predicts that a plot of CT/CW(1 - ) vs. CW(1 - ) will be linear with a slope of 2KM2KD and an intercept of KM. The value of of used in Eq.(9) is obtained from the known value of Ka. Ka = PROCEDURE: A. Preparation of the toluene/benzoic acid/water mixtures Eight mixtures of toluene/benzoic acid/water are to be studied so that the distribution can be observed over a wide range of concentration. These mixtures are to be made according to Table I, using the burettes for toluene and the benzoic acid solution in toluene, the water should be added using the 50.00 mL pipette. Make up the mixtures in the stoppered bottles and allow the equilibrium distribution to be attained over a period of about 30 minutes, shaking frequently. During the equilibration period, practice the two titrations involved on samples of benzoic acid in toluene and in water as supplied in the laboratory. TABLE I Mixture Benzoic acid in toluene 50 mL 45 mL 40 mL 35 mL 30 mL 25 mL 20 mL 15 mL Toluene Water
2CW
1-
= 6.3 x 10-5 for benzoic acid at 20C
I II III IV V VI VII VIII
+ 0 mL + 5 mL + 10 mL + 15 mL + 20 mL + 25 mL + 30 mL + 35 mL
+ 50 mL + 50 mL + 50 mL + 50 mL + 50 mL + 50 mL + 50 mL + 50 mL
-3At the end of the equilibration period, allow the mixture to separate into two layers. Decant the upper toluene layer into a large, clean, dry test tube, and cork it at once. (This separation need not be perfect). B. Titration of the toluene layer Pipette 15.00 mL of the toluene solution from the upper layer in the test tube into a clean (not necessarily dry) Erlenmeyer flask. WIPE THE OUTSIDE OF THE PIPETTE BEFORE ADJUSTING THE TRANSFER VOLUME TO AVOID CARRYING OVER ANY EXTRA, UNMEASURED SOLUTION. Pipette 25.00 mL of the 0.030 M NaOH and titrate with 0.0200 M HCl (Record exact concentrations) until the pink color of the phenolphthalein indicator disappears (this is the end point for the titration. Since neutralization of the benzoic acid in the toluene layer with the NaOH in aqueous solution is slow because of the immiscibility of the two layers, the two layers of solution must be stoppered and well shaken before the back titration with the 0.020 M HCl. NOTE: Stir slowly when doing the titration because the low molarity of NaOH leads to problems with CO2 absorption causing the end point to be reached faster than expected. The CO2 forms carbonic acid which causes the weak solution of NaOH to be neutralized. Repeat the titration, cleaning the pipette between samplings. Follow the same procedure in duplicate for each of the other eight solutions and record your titration values on the blackboard in the laboratory for other students to record. C. Titration Blank for the toluene layer This is a necessary correction to the titration in part B due to the fact that CO2 is absorbed and reacts with the dilute NaOH added when the aqueous & toluene layers are well shaken before back titration with the HCl solution. Pipette 15.00 mL of pure toluene into a clean Erlenmeyer flask. Pipette 25.00 mL of the 0.030 M NaOH, stopper and shake well for the same period of time as you did for each of your titrations in part B. Titrate with the 0.0200 M HCl in exactly the same fashion as you did in part B. Repeat the titration with a second sample. D. Titration of the aqueous layer Titrate 20.00 mL of the lower, aqueous layer with the 0.006 M NaOH. The lower, aqueous layer must be reached through the upper, toluene layer. This is best done by blowing gently through the pipette as it is inserted to ensure that none of the toluene layer enters. Transfer the 20.00 mL to a clean Erlenmeyer flask, wipe the pipette before adjusting the transfer volume as before. Since the concentration of the benzoic acid in the water layer will be low, expect small amounts of titrant to be used in this titration. Repeat the titration. Follow the same procedure in duplicate for the other eight solutions and record your titration values on the blackboard in the laboratory for other students to record.
-4CALCULATIONS: 1. Calculate how many mL of 0.020 M HCl should have been used to neutralize the 25.00 mL of 0.030 M NaOH in the titration blank of the toluene layer (Part C). The difference between the calculated volume and the actual volume it took to do the titration is the correction factor which must be added to each of your titration volumes for the toluene layer titrations of solution I to V. From the volumes of titrant used for each solution you can calculate the concentration of benzoic acid in the toluene and water layers, CT and CW respectively. You should also calculate the total amount benzoic acid present in each mixture (I to VIII). You should find that the amount of benzoic acid present follows the ratios you placed in mixtures I to VIII. From equation (10), calculate the value of for each value of CW. You should tabulate your results under the following headings: solution number, mL of HCl used for toluene layer, CT, mL of NaOH used for water layer, CW, (CT/CW), , CW(1 - ) and CT/CW(1 - ). NOTE: You should show one complete calculation in your lab report. All of the data point obtained by the class can be input into the spread sheet on the computer which will automatically calculate the above values. 5. Plot all of the individual values of CT/CW(1 - ) vs. CW(1 - ) obtained by the class and from this plot obtain tbe value KM(intercept) and KD (which can be obtained from the slope of this line as indicated in the introduction). USE THE LINEAR REGRESSION PROGRAM ON YOUR CALCULATOR OR ON THE COMPUTER IN THE LAB TO OBTAIN THIS PLOT AS WELL AS THE BEST VALUES FOR THE SLOPE AND INTERCEPT. You should also calculate the concentration of benzoic acid in tbe original toluene solution used (i.e. 4.5 g/L).
2.
3.
4.
6.
QUESTIONS: 1. 2. Comment on the size of these equilibrium constants (i.e. what do they imply about the system?). Suggest a reason for the fact that benzoic acid exists as a monomer in aqueous solution but mainly as a dimer in toluene. Draw the structure of the benzoic acid dimer. Suggest how this experiment could be extended so that we might obtain information as to the H and S for the dimerization of benzoic acid in toluene as well as for the distribution of benzoic acid monomers between toluene and water.
3. 4.
REFERENCES: 1. 2. 3. E.A. Moelwyn-Hughes, J.Chem.Soc., 850 (1940). A.K.M. Shamsul Huq and S.A.K. Lodhi, J. Phys. Chem., 70, 1354(1966). M. Davies, P. Jones, D. Patnaik and E.A. Moelwyn-Hughes, J. Chem. Soc., 1249 (1951).
You might also like
- Determine An Equilibrium ConstantDocument13 pagesDetermine An Equilibrium ConstantMeMeMelol100% (2)
- 2011 ACJC H2 Chem P1,2 AnswersDocument15 pages2011 ACJC H2 Chem P1,2 Answersonnoez100% (1)
- MT Lab Final RecordDocument85 pagesMT Lab Final RecordPRABATH MADHAVANNo ratings yet
- Mass Transfer With/Without Chemical Rection (Solid-Liquid System)Document7 pagesMass Transfer With/Without Chemical Rection (Solid-Liquid System)Sameep JainNo ratings yet
- Mass Transfer With/Without Chemical Rection (Solid-Liquid System)Document7 pagesMass Transfer With/Without Chemical Rection (Solid-Liquid System)Sameep JainNo ratings yet
- Determining Equilibrium Constants (KcDocument6 pagesDetermining Equilibrium Constants (KcNur Farhana LukhmanNo ratings yet
- Manual For Calculating Distribution CoefficientDocument3 pagesManual For Calculating Distribution CoefficientMohit JagtapNo ratings yet
- CHEM 334L - Conductance of Solutions - Estimating K For A Weak AcidDocument4 pagesCHEM 334L - Conductance of Solutions - Estimating K For A Weak Acidfdobonat613100% (1)
- Mixture of Carbonate BicarbonateDocument9 pagesMixture of Carbonate BicarbonateIan Justine SanchezNo ratings yet
- Objective Theory Apparatus Procedure Result Sample of Calculation Discussion Conclusion Recommendation Reference AppendicesDocument19 pagesObjective Theory Apparatus Procedure Result Sample of Calculation Discussion Conclusion Recommendation Reference Appendicesahmad pidotNo ratings yet
- Equilibrium Constant PupilDocument13 pagesEquilibrium Constant PupilReinaldo RaymondNo ratings yet
- Equilibrim ConstantDocument5 pagesEquilibrim ConstantArchibald MiguelNo ratings yet
- Experiment P01 Determination of The Equilibrium Constant For Esterification (Ethanoic Acid and Propan-1-Ol)Document4 pagesExperiment P01 Determination of The Equilibrium Constant For Esterification (Ethanoic Acid and Propan-1-Ol)Shirley SayNo ratings yet
- Preparing Rum-Scented Ester: Kinetics and Titration StudyDocument4 pagesPreparing Rum-Scented Ester: Kinetics and Titration StudyMJ TarhiniNo ratings yet
- Titration Lab ReportDocument5 pagesTitration Lab ReportvaiNo ratings yet
- TK-315 CPI2 - 1 - NonReacting SystemsDocument37 pagesTK-315 CPI2 - 1 - NonReacting SystemsIndahNo ratings yet
- Preparation For Chemistry TA3 (Practical)Document9 pagesPreparation For Chemistry TA3 (Practical)leekicia123No ratings yet
- DistributionDocument4 pagesDistributionJessa Suñga50% (2)
- Experiment 3 Three Component SystemsDocument8 pagesExperiment 3 Three Component Systemsmohammednoor_No ratings yet
- CPS410 Exam 2013Document3 pagesCPS410 Exam 2013KarinaNo ratings yet
- Chemical Equilibrium Lab 2012Document4 pagesChemical Equilibrium Lab 2012Untung Ari Wibowo100% (1)
- Exercise 2 Partition Coefficient of Succinic Acid PDFDocument4 pagesExercise 2 Partition Coefficient of Succinic Acid PDFKeziaNo ratings yet
- Record Book PCDocument13 pagesRecord Book PCSmitNo ratings yet
- TK-315 CPI2 - 1 - NonReacting SystemsDocument34 pagesTK-315 CPI2 - 1 - NonReacting SystemsMuhammad IkbalNo ratings yet
- Official Sample 2016Document8 pagesOfficial Sample 2016jassem danafNo ratings yet
- Lab Manual B.Tech Chemistry 2022Document27 pagesLab Manual B.Tech Chemistry 2022PRATYAKSHA SHEKHARNo ratings yet
- EXP5procedure PDFDocument2 pagesEXP5procedure PDFGeneva OrañoNo ratings yet
- Class 12th For Board ExamDocument5 pagesClass 12th For Board Examakashsadoriya5477No ratings yet
- TK-315 CPI2 - 1 - NonReacting SystemsDocument33 pagesTK-315 CPI2 - 1 - NonReacting SystemsBayu Purnama Ridjadi75% (4)
- SaponificationDocument4 pagesSaponificationSihanu Subasingha0% (1)
- CHE 101 Assignment 2023Document1 pageCHE 101 Assignment 2023donmichael027No ratings yet
- HW 2Document1 pageHW 2Lee WotNo ratings yet
- 254 8 Liquid Vapour EquilibriumDocument6 pages254 8 Liquid Vapour EquilibriumJustina JankauskaitėNo ratings yet
- Phase Equilibrium WorksheetDocument15 pagesPhase Equilibrium WorksheetJue MayaNo ratings yet
- 1st-Year-Titration PRACTICALDocument9 pages1st-Year-Titration PRACTICALArundhuti Sinha RoyNo ratings yet
- Experiment 4 - Distribution (Formal Report)Document5 pagesExperiment 4 - Distribution (Formal Report)joanne_blanco100% (6)
- Chapter 15 Test BankDocument41 pagesChapter 15 Test BankMeowCat12345678950% (2)
- Lubna - Chemistry - 12 LSDocument4 pagesLubna - Chemistry - 12 LSkhattab994No ratings yet
- CHM 471 Tutorial 3 Phase DiagramDocument4 pagesCHM 471 Tutorial 3 Phase DiagramCharlesRolendNo ratings yet
- Liquid-Liquid Extraction ExperimentDocument23 pagesLiquid-Liquid Extraction ExperimentSameep JainNo ratings yet
- Winter 2010 Test #2 Buffer QuestionsDocument20 pagesWinter 2010 Test #2 Buffer QuestionsDavidNo ratings yet
- Set 3 AnsDocument13 pagesSet 3 Ansluis fernando santos narvaezNo ratings yet
- CHEMISTRY 12: VOLUMETRIC ANALYSIS & TITRATIONSDocument24 pagesCHEMISTRY 12: VOLUMETRIC ANALYSIS & TITRATIONSJoaquinNo ratings yet
- Physical Chemistry Laboratory I Experiment 3 Effect of Ionic Strength On The Solubility of CasoDocument6 pagesPhysical Chemistry Laboratory I Experiment 3 Effect of Ionic Strength On The Solubility of CasorajNo ratings yet
- Chemical Equilibrium: CHE 195 Process ChemistryDocument25 pagesChemical Equilibrium: CHE 195 Process ChemistryMohd Shahrul Nizam SallehNo ratings yet
- Le Chatelier's Principle and Equilibrium: DiscussionDocument7 pagesLe Chatelier's Principle and Equilibrium: DiscussionShyweyNo ratings yet
- Gas Liquid AbsorptionDocument9 pagesGas Liquid AbsorptionShashwat OmarNo ratings yet
- Theoretical and Practical Considerations of Newer Methods for Assay of Halide SaltsDocument5 pagesTheoretical and Practical Considerations of Newer Methods for Assay of Halide SaltsDavi Abreu Carvalho MotheNo ratings yet
- Kinetics 1Document3 pagesKinetics 1JuarezNo ratings yet
- EquilibriumDocument5 pagesEquilibriumShafeeq IbraheemNo ratings yet
- Problems Analytical ChemistryDocument7 pagesProblems Analytical ChemistryQuyen BuiNo ratings yet
- Distillation: Unit Operations (Che 347/ 251)Document27 pagesDistillation: Unit Operations (Che 347/ 251)Amira KormainNo ratings yet
- 6-Volumetric AnalysisDocument48 pages6-Volumetric AnalysisOmar EzzatNo ratings yet
- Experiment No. 4: Adamson University College of Engineering Chemical Engineering DepartmentDocument10 pagesExperiment No. 4: Adamson University College of Engineering Chemical Engineering DepartmentRon PascualNo ratings yet
- Solution Markswise QuestionsDocument9 pagesSolution Markswise QuestionsSachin GuptaNo ratings yet
- Tray Distillation Column With RefluxDocument26 pagesTray Distillation Column With RefluxMelvin MoorNo ratings yet
- Solutions Assignment-1Document3 pagesSolutions Assignment-1Akshara SreeNo ratings yet
- Expt01 HCL and NaOH AnsDocument3 pagesExpt01 HCL and NaOH AnsaragpdNo ratings yet
- Gas Hydrates 1: Fundamentals, Characterization and ModelingFrom EverandGas Hydrates 1: Fundamentals, Characterization and ModelingDaniel BrosetaNo ratings yet
- Physical Chemistry of Polyelectrolyte SolutionsFrom EverandPhysical Chemistry of Polyelectrolyte SolutionsMitsuru NagasawaNo ratings yet
- Orifice Plate ReportDocument1 pageOrifice Plate ReportdsdeNo ratings yet
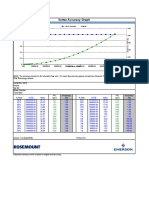
- Vortex Accuracy Graph: Flow Technology AdvisorDocument1 pageVortex Accuracy Graph: Flow Technology AdvisordsdeNo ratings yet
- Calculos Lab KpsDocument4 pagesCalculos Lab KpsdsdeNo ratings yet
- JHFGDocument1 pageJHFGdsdeNo ratings yet
- Calve GuitarraDocument1 pageCalve GuitarradsdeNo ratings yet
- Pyramid SongDocument2 pagesPyramid SongdsdeNo ratings yet
- DsewDocument2 pagesDsewdsdeNo ratings yet
- 4658Document2 pages4658dsdeNo ratings yet
- Calve GuitarraDocument1 pageCalve GuitarradsdeNo ratings yet
- TsDocument1 pageTsdsdeNo ratings yet
- How Light Affects Plant TranspirationDocument7 pagesHow Light Affects Plant TranspirationG100% (1)
- Sampling DistributionDocument30 pagesSampling Distributionsemhal gebremedhinNo ratings yet
- SINHGAD TECHNICAL EDUCATION SOCIETY’S SINHGAD INSTITUTE OF TECHNOLOGY LAB MANUALDocument41 pagesSINHGAD TECHNICAL EDUCATION SOCIETY’S SINHGAD INSTITUTE OF TECHNOLOGY LAB MANUALrohit100% (1)
- Nikola Tesla's Colorado Springs NotesDocument431 pagesNikola Tesla's Colorado Springs Notesanon-377478100% (20)
- WMA11-01 Que 20220513Document20 pagesWMA11-01 Que 20220513Karl HIRMASNo ratings yet
- Balanced Scorecard & Transfer Pricing TechniquesDocument2 pagesBalanced Scorecard & Transfer Pricing TechniquesMeghan Kaye LiwenNo ratings yet
- ML B 200 Bkasr183Document34 pagesML B 200 Bkasr183Marco Antonio PrietoNo ratings yet
- Transitioning Residential Neighbourhoods: A Case Study of Jayalaximpuram, Mysore, IndiaDocument5 pagesTransitioning Residential Neighbourhoods: A Case Study of Jayalaximpuram, Mysore, IndiaRAJIV VINOD CONJEEVARAM 1961714No ratings yet
- The Alchemist Study GuideDocument5 pagesThe Alchemist Study GuideSK ElangovanNo ratings yet
- The Group Actions of MuscleDocument21 pagesThe Group Actions of MuscleMuhammad Owais qarniNo ratings yet
- CALCULUS 1: INTRODUCTION TO LIMITSDocument30 pagesCALCULUS 1: INTRODUCTION TO LIMITSYvette CajemeNo ratings yet
- Design-Related Research in Landscape ArchitectureDocument20 pagesDesign-Related Research in Landscape ArchitectureHoang PhungNo ratings yet
- Icest 2011 977 930Document8 pagesIcest 2011 977 93001666754614No ratings yet
- Vietnamese Synonyms and Antonyms Practice TestDocument9 pagesVietnamese Synonyms and Antonyms Practice TestHà Kylur'xNo ratings yet
- (PDF) Thermally Sprayed Solder - Braze Filler Alloys For The Joining of Light MetalsDocument27 pages(PDF) Thermally Sprayed Solder - Braze Filler Alloys For The Joining of Light MetalsKewell LimNo ratings yet
- GL XX Mobil DTE Oil Double Letter SeriesDocument3 pagesGL XX Mobil DTE Oil Double Letter SeriesMuhammad RipandiNo ratings yet
- Kuhn's View of Scientific Revolutions as Paradigm ShiftsDocument19 pagesKuhn's View of Scientific Revolutions as Paradigm ShiftsEli EliNo ratings yet
- Alya Mawriza Siregar - 1181002025 - Uas Auditing Ii - Akt62Document9 pagesAlya Mawriza Siregar - 1181002025 - Uas Auditing Ii - Akt62Alya mawrizaNo ratings yet
- Albertus Magnus - The Grand Albert Admirable SecretsDocument130 pagesAlbertus Magnus - The Grand Albert Admirable SecretsIsrael Vargas Rosado100% (1)
- PR2 - Writing HypothesisDocument18 pagesPR2 - Writing HypothesisRowell Marquina100% (1)
- D9520-069 882 - 2015 - 10 - 14 - Bal - enDocument55 pagesD9520-069 882 - 2015 - 10 - 14 - Bal - enmgustavo5No ratings yet
- Manual of Forever Living ProductsDocument105 pagesManual of Forever Living Productsspiritualbeing100% (20)
- Stack-up Simulator Revision HistoryDocument4 pagesStack-up Simulator Revision HistoryChristianNo ratings yet
- Project Proposal - First Aid SeminarDocument2 pagesProject Proposal - First Aid SeminarAngelo FaustinoNo ratings yet
- Hasil SPSSDocument12 pagesHasil SPSSHAIDAR ADI NUGROHO 1No ratings yet
- Lecture 19 - Plane Wave Expansion MethodDocument24 pagesLecture 19 - Plane Wave Expansion MethodZhenhua HuangNo ratings yet
- Probability and StatisticsDocument90 pagesProbability and StatisticsNala A.100% (1)
- Chapter 1 5Document66 pagesChapter 1 5Jennylyn BraceroNo ratings yet
- Motivation and PerformanceDocument15 pagesMotivation and PerformanceRizqina AwliyaNo ratings yet
- Lunes Martes Miyerkules Huwebes: GRADES 1 To 12 Daily Lesson LogDocument5 pagesLunes Martes Miyerkules Huwebes: GRADES 1 To 12 Daily Lesson Logritz manzanoNo ratings yet