Professional Documents
Culture Documents
Detection and Quantification of Histamine and Iron
Uploaded by
Marc Eric Redondo0 ratings0% found this document useful (0 votes)
58 views1 pageDetection and Quantification of Histamine and Iron
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentDetection and Quantification of Histamine and Iron
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
58 views1 pageDetection and Quantification of Histamine and Iron
Uploaded by
Marc Eric RedondoDetection and Quantification of Histamine and Iron
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 1
DETECTION AND QUANTIFICATION OF HISTAMINE AND IRON (Fe) IN THREE VARIETIES OF TUNA
Janne Paulyne P. Libunao, Kristoffer John M. Nerona & Janel R. Cacuyog
Introduction
Fish is widely consumed in many parts of the world by humans because it has high protein content, low saturated fat and also contains omega 3 fatty acids known
to support good health. Histamine fish poisoning, an entirely preventable condition, is among the most common toxicities related to fish ingestion. Histamine fish poisoning,
which had been previously termed scombroid fish poisoning, pseudo allergic fish poisoning, histamine overdose, or mahi-mahi flush, accounts for less than 0.5% of all food-
borne outbreaks reported to the Centers for Disease Control and Prevention (CDC) and 37% of all seafood-related food-borne illnesses. It presents as a possible allergic
reaction after consuming certain fish but is actually caused by ingesting toxins within the fish's tissues .Biologically active substance found in a great variety of living
organisms is histamine. It is distributed widely, albeit unevenly, throughout the animal kingdom and is present in many plants and bacteria and in insect venom. It is formed
by the decarboxylation (the removal of a carboxyl group) of the amino acid histidine. Iron is a trace element of considerable concern in public health. A complete, accurate
and quantitative knowledge of the levels and forms (heme or nonheme) of iron in foods is important since the bioavailability of each type of iron differs. Fish is a major
source of iron for adults and children. Iron deficiency causes anemia. Meat is the main source of heme iron in human diets, and meat also makes a large contribution to the
nonheme iron content of human diets. Levels of total, nonheme and heme iron are often determined in meats, but little effort has been spent on validating the methods used
to analyze iron. Most types of fish contain iron, providing up to about 10 percent of the RDA in an average serving. For example, a 6-ounce serving of canned salmon or
tuna contains about 1.5 milligrams of iron. Other varieties of fish also provide iron, including cod, with 0.8 milligrams in 6 ounces; flounder, with 0.6 milligrams in 6 ounces;
and haddock, with 0.4 milligrams in a similar-sized serving. Both wild-caught and farmed fish supply iron. Our body uses iron in many ways. One of the most important uses
is for manufacturing heme, an iron-containing compound that binds oxygen. As part of hemoglobin, heme carries iron in your blood to all parts of your body. Iron is also part
of myoglobin, another oxygen-carrying compound found in your muscles. Besides its function in oxygen transport, iron is also necessary for the action of many enzymes.
Our body needs the mineral iron for many extremely important functions, including helping your red blood cells carry oxygen to all your cells and tissues. A lack of iron is the
most prevalent nutrient deficiency in America, according to the Linus Pauling Institute. You can increase your intake of this vital micronutrient by adding iron-rich seafood to
your diet.
Statement of the Problem
The study aimed to detect and quantify
Histamine and Iron in Three Varieties of Tuna.
Specifically, it answered the following
questions:
1.) Which of the following varieties has
the most percentage of Histamine
and Iron?
2.1) Blue fin Tuna
2.2) Yellow fin Tuna
2.3) Big eye Tuna
2.) What are the factors affecting
Histamine and Iron level in the three
varieties of Tuna?
3.) What percentage of the Tuna is
positive with Iron and Histamine?
What percentage of Tuna is negative
with Iron and Histamine?
4.) Is there a significant difference in the
levels of Histmine and Iron among
the three varieties of Tuna?
Methodology
Preparation of Materials The fresh raw
tuna and histamine dihydrochloride will be bought
at the Microbiology Laboratory of Notre Dame of
Dadiangas University Main Campus. The
Histamine Dihydrochloride of 1mM acetonitrile
solution of 2, 3- naphathalene dicarboxylaldehyde
will also be prepared. Fresh raw tuna will be
weighed 5 g. The laboratory equipments to be
used include the following: filter paper discs, test
tubes, petri dishes, injection syringe. Gathering
and Preparation of Samples The experiment wil
be performed by addition of a histamine standard
preparation to a real sample of fresh fish meat.
The histamine standard preparation used was
histamine dihydrochloride, and the histamine
detection reagent used was a 1mM acetonitrile
solution of 2, 3- naphathalene dicarboxylaldehyde.
Preparation of Fresh Raw Tuna The procedure
weighed 5g of fresh raw tuna in a homogenizer,
added 25 ppm, 50 ppm, and 100 ppm aqueous
solutions of the histamine standard preparation
and 30 ml of a 5% aqueous TCA solution, and
homogenized the respective mixtures for 1 minute.
After centrifugation of each of the homogenized
solutions at 4 C the respective mixtures for 1
minute. After centrifugation of each of the
homogenized solutions at 4 C., 1000 rpm, 1000 l
of the supernatant was mixed with 250 l of a 1 M
sodium hydroxide solution with stirring to give a
TCA-extracted sample solution. The TCA-
extracted sample solutions had pH of 8. Methanol-
extracted sample solutions were prepared by the
similar procedure with replacement of the aqueous
TCA solution by methanol and with addition of 20
l of the 1 M sodium hydroxide solution in place of
250 l. The methanol-extracted sample solutions
had pH of 7. A. Histamine detection method
The procedure introduced 500 l of each sample
solution to the histamine detection cartridge and
applied pressure to the carrier with a piston of the
injection syringe to make the sample solution pass
through the carrier. The procedure then similarly
introduced 500 l of each sample solution to the
cartridge under pressure to make the sample
solution pass through the carrier, sequentially
introduced one 200 l aliquot of a 0.2 M phosphate
buffer (pH 6.0) and four 200 l aliquots of water to
the cartridge to wash the carrier, and subsequently
introduced 200 l of the histamine detection
reagent to make the reagent pass through the
carrier in the similar manner. B. Total Iron Method
Tuna samples will be accurately weighed into 125
mL Erlenmeyer flasks and 15 mL of concentrated
nitric acid will be added. Each flask will be left to
predigest at room temperature for 4-6 hours or
overnight. The flask will be placed on a hot plate
set at 100C until dry. Hydrogen peroxide sulfuric
reagent containing peroxymonosulfuric acid, will be
added in 1 mL aliquots to each sample until they
all become clear, typically after three or four
additions. The flask will be left on the hot plate until
all peroxide will be expelled and the white vapors
of sulfuric acid become evident. The clear digest
will allow to cool and quantitatively transferred to
10 mL volumetric flask using 0.01 N HCl as the
rinse. Quantitative Analysis In order to determine
the absorbance value of histamine and the iron in
tuna, the data will be subjected to quantitative
analysis.
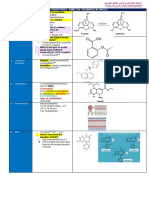
Method Histamine
T1
T1 Histamine Dihydrocholride
T2 Total Iron Method
R1- Big Eye Tuna
R2- Yellow Fin Tuna
R3- Blue Fin Tuna
Figure 1. Research Design
Absorbance
Value
R1
Detection and
Quantification R2
R3
T2
R1
R2
R3
Iron
Detection and
Quantification
Absorbance
Value
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- Canadian SolarDocument4 pagesCanadian SolarMarc Eric RedondoNo ratings yet
- Ifrs 1Document6 pagesIfrs 1Marc Eric RedondoNo ratings yet
- IFRS For SMEs PDFDocument20 pagesIFRS For SMEs PDFMarc Eric RedondoNo ratings yet
- Ifrs 15Document58 pagesIfrs 15furqanNo ratings yet
- Theory of AccountsDocument11 pagesTheory of AccountsMarc Eric Redondo50% (2)
- SIC 29 - Disclosure - Service Concession ArrangementsDocument6 pagesSIC 29 - Disclosure - Service Concession ArrangementsJimmyChaoNo ratings yet
- IFRS 14 Regulatory Deferral AccountsDocument24 pagesIFRS 14 Regulatory Deferral AccountsMarc Eric RedondoNo ratings yet
- SIC 32 - Intangible Assets - Website CostsDocument6 pagesSIC 32 - Intangible Assets - Website CostsJimmyChaoNo ratings yet
- Sic 25Document4 pagesSic 25Stephanie GumapacNo ratings yet
- SIC 07 - Introduction of The EuroDocument4 pagesSIC 07 - Introduction of The EuroJimmyChaoNo ratings yet
- SIC 10 - Government Assistance - No Specific Relation To Operating ActivitiesDocument4 pagesSIC 10 - Government Assistance - No Specific Relation To Operating ActivitiesJimmyChaoNo ratings yet
- Interview Evaluation Rubric: I. Application Forms and Recommendation FormDocument1 pageInterview Evaluation Rubric: I. Application Forms and Recommendation FormMarc Eric RedondoNo ratings yet
- Intangible AssetsDocument4 pagesIntangible AssetsMarc Eric RedondoNo ratings yet
- SSS, PhilHealth, Pag-IBIG Contribution SchedulesDocument1 pageSSS, PhilHealth, Pag-IBIG Contribution SchedulesMarc Eric RedondoNo ratings yet
- Deductions From Gross EstateDocument3 pagesDeductions From Gross EstateMarc Eric Redondo100% (5)
- MAS Practice StandardsDocument6 pagesMAS Practice StandardsMarc Eric RedondoNo ratings yet
- Enron and WorldCom ScandalDocument5 pagesEnron and WorldCom ScandalMarc Eric RedondoNo ratings yet
- Rules On LeaveDocument9 pagesRules On LeaveAnonymous 1lYUUy5T89% (9)
- 4GACPA 2013.chart of Accounts - UacsDocument20 pages4GACPA 2013.chart of Accounts - UacsMarc Eric RedondoNo ratings yet
- Digestive SystemDocument5 pagesDigestive SystemMarc Eric RedondoNo ratings yet
- The Evolution of CellphonesDocument2 pagesThe Evolution of CellphonesMarc Eric RedondoNo ratings yet
- Alphabetical List of PhobiasDocument9 pagesAlphabetical List of PhobiasMarc Eric RedondoNo ratings yet
- CatholicismDocument2 pagesCatholicismMarc Eric RedondoNo ratings yet
- Boyle's Law: PV Constant. See Also GasesDocument2 pagesBoyle's Law: PV Constant. See Also GasesMarc Eric Redondo100% (1)
- Registry of Appropriations and AllotmentsDocument1 pageRegistry of Appropriations and AllotmentsMarc Eric RedondoNo ratings yet
- OPDDocument2 pagesOPDMarc Eric RedondoNo ratings yet
- Curriculum Vitae Replace With First Name(s) Surname(s)Document2 pagesCurriculum Vitae Replace With First Name(s) Surname(s)Valentina100% (1)
- Bureau of The Treasury: Registry of Notice of Cash Allocation and ReplenishmentsDocument1 pageBureau of The Treasury: Registry of Notice of Cash Allocation and ReplenishmentsMarc Eric RedondoNo ratings yet
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (894)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- StructuresDocument7 pagesStructuresFarouk Ramadan100% (1)
- Fish Gelatin-Chitosan Films Improved with PlasticizersDocument6 pagesFish Gelatin-Chitosan Films Improved with PlasticizersValentina RoznovNo ratings yet
- Synthesis and Application of Eosin: Kabeer Fatima, Sofia Nosheen, Humera and Munazza AzharDocument7 pagesSynthesis and Application of Eosin: Kabeer Fatima, Sofia Nosheen, Humera and Munazza AzharPetr Svoboda67% (3)
- Dewar BenzeneDocument7 pagesDewar BenzenechinuasfaNo ratings yet
- Quiz Organic 1Document6 pagesQuiz Organic 1ronakgupta332005No ratings yet
- Binyam Kebede 2013 ThesisDocument93 pagesBinyam Kebede 2013 ThesisPastor MogollónNo ratings yet
- ghsc1528 PDFDocument44 pagesghsc1528 PDFSiddharth SahuNo ratings yet
- Saboor Khalid Et Al. 2021. JAEFSECDocument41 pagesSaboor Khalid Et Al. 2021. JAEFSECSaboor OfficialNo ratings yet
- CODEX STANDARD FOR FERMENTED MILKSDocument11 pagesCODEX STANDARD FOR FERMENTED MILKSvabimhah100% (2)
- CN100371313C - Process For Preparing O-Trifluoromethyl Aniline - Google PatentsDocument13 pagesCN100371313C - Process For Preparing O-Trifluoromethyl Aniline - Google PatentsyoumasankarNo ratings yet
- AfsDocument35 pagesAfsnora santiNo ratings yet
- Biomolec ConceptMaps CHO, Lipid, ProtDocument4 pagesBiomolec ConceptMaps CHO, Lipid, ProtLorence ArnedoNo ratings yet
- Amphetamine Synthesis Very EasyDocument3 pagesAmphetamine Synthesis Very EasyG Moran83% (23)
- CompotecLine Hoses OIL800Document2 pagesCompotecLine Hoses OIL800rendy_perdana3No ratings yet
- Gacan Qabad Chemistry Book With AnswersDocument32 pagesGacan Qabad Chemistry Book With Answerscazmi Andirahman100% (1)
- Thrope Ziegler Cyclization SearchDocument4 pagesThrope Ziegler Cyclization SearchahmedramadanNo ratings yet
- SugarcaneDocument83 pagesSugarcaneabrahanNo ratings yet
- NSEC Solved Paper 2010Document7 pagesNSEC Solved Paper 2010whatismyusername1947No ratings yet
- Ariola Bschem4a Foodchempc MidtermDocument3 pagesAriola Bschem4a Foodchempc Midtermjohn buenafeNo ratings yet
- Chapter 12 & 13: Energy, respiration and photosynthesisDocument88 pagesChapter 12 & 13: Energy, respiration and photosynthesiseric sivaneshNo ratings yet
- Wa0001.Document11 pagesWa0001.Bhagyesh KhobragadeNo ratings yet
- Expansion Joint Waterstops, Internal:, Black, LECOTRIL DIN 18541Document2 pagesExpansion Joint Waterstops, Internal:, Black, LECOTRIL DIN 18541Dilhara WickramaarachchiNo ratings yet
- AcetoneDocument7 pagesAcetoneGeorgiana AndreeaNo ratings yet
- Eudragit Application Guidelines PDFDocument2 pagesEudragit Application Guidelines PDFAustinNo ratings yet
- Journal of Bbiological Science - Identification of Effective Organic Carbon For Biofloc Shrimp Culture SystemDocument7 pagesJournal of Bbiological Science - Identification of Effective Organic Carbon For Biofloc Shrimp Culture SystemReno MurdaNo ratings yet
- Analytical Methods For Lipases Activity Determination - A ReviewDocument8 pagesAnalytical Methods For Lipases Activity Determination - A ReviewgotcanNo ratings yet
- Carbon Black Feed StockDocument2 pagesCarbon Black Feed StockdivyaNo ratings yet
- Organic Chemistry Naming GuideDocument6 pagesOrganic Chemistry Naming GuideNelzen GarayNo ratings yet
- Alergia BavsvebsiDocument16 pagesAlergia BavsvebsiNana OkujavaNo ratings yet
- Heterocyclic CompoundsDocument26 pagesHeterocyclic Compounds29decNo ratings yet