Professional Documents
Culture Documents
Map Reaction
Uploaded by
Naguib ZakariaCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Map Reaction
Uploaded by
Naguib ZakariaCopyright:
Available Formats
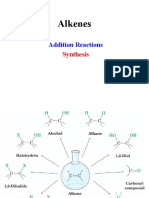
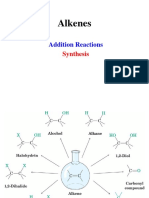
REACTION MAP FOR CARBON COMPOUND
EXAMPLE : ETHANOL
H H Fermentation Process
| | C6H12O6 2CH3CH2OH + 2CO2
H–C–C–H o
| | Temp : 18 – 20 C
H H H Br H H Catalyst : zymase from yeast
| | | |
H–C – C–H Other condition : absence of O2
H–C–C–H | |
| | OH OH
Br Br
+ HBr gas
+ Br2 aqueous + KmnO4 acidified
Halogenation
ETHANE Hydrogenation Process ETHENE Hydration Process ETHANOL Oxidation Process ETHANOIC ACID
CH3CH3 + H2 CH2CH2 + H2O (gas) CH3CH2OH + KmnO4 acidified (H2SO4) // CH3COOH
o o
Nickel as catalyst, 180 C H3PO4 as catalyst, 300 C, 60 atm + K2Cr2O7 acidified (H2SO4)
+ Br2 aqueous (UV)
Substitution Process
Dehydration Process
Esterification Process
+ H2SO4 as catalyst
Br Br
| |
Br – C – C – Br
| | ETHYL ETHANOATE
Br Br CH3COOCH2CH3
Prepared by MNZ©
You might also like
- Organic TuteDocument1 pageOrganic TuteDimuthu SandaruwanNo ratings yet
- Exercise - VI (B) JEE-Problems: Solution Slot - 3 (Chemistry)Document1 pageExercise - VI (B) JEE-Problems: Solution Slot - 3 (Chemistry)Priyanshu RajNo ratings yet
- Al KynesDocument15 pagesAl KynesAbdullah AmjadNo ratings yet
- Elimination ReactionDocument22 pagesElimination ReactionSabitry YadavNo ratings yet
- Peta Minda KimiaDocument36 pagesPeta Minda KimiaNATASHA 'ALIA BINTI ZULKIFLINo ratings yet
- Alkenes ReactionsDocument69 pagesAlkenes ReactionsHamidatun NisaNo ratings yet
- 28 Aldehydes Ketones Formula Sheets QuizrrDocument8 pages28 Aldehydes Ketones Formula Sheets Quizrrrakeshnayak78487No ratings yet
- Matriculation Chemistry (Amino Acids) Part 2Document10 pagesMatriculation Chemistry (Amino Acids) Part 2ridwanNo ratings yet
- Alkenes ReactionsDocument69 pagesAlkenes ReactionsAhmad SayyedahmadNo ratings yet
- All Named ReactionsDocument3 pagesAll Named ReactionsSamrathsingh Hayer100% (1)
- Carboxylic Acids:: R-Cooh, R-Co HDocument43 pagesCarboxylic Acids:: R-Cooh, R-Co HmacybnzNo ratings yet
- Obtención de Alcoholes por Sustitución Nucleófila y Reactivos de GrignardDocument7 pagesObtención de Alcoholes por Sustitución Nucleófila y Reactivos de GrignardMARITZA QUISPE VILLARREALNo ratings yet
- OCHEMDocument9 pagesOCHEMLoraNo ratings yet
- Organic ChemistryDocument7 pagesOrganic ChemistryABDULLAH SHAHZADNo ratings yet
- Organic Name ReactionsDocument2 pagesOrganic Name ReactionsShruti MohrilNo ratings yet
- Alkynes. C: C H H:C:::C:H H-C C-HDocument26 pagesAlkynes. C: C H H:C:::C:H H-C C-HLalaxx D'BlacklistNo ratings yet
- Flow Chart - HydrocarbonsDocument77 pagesFlow Chart - HydrocarbonsKalyan Reddt100% (2)
- +2 NEET IntelliQuest PCB-3 (28.01.2021) AnsKey - 10645292Document3 pages+2 NEET IntelliQuest PCB-3 (28.01.2021) AnsKey - 10645292swap2005sharmaNo ratings yet
- GOC Class11thDocument38 pagesGOC Class11thAnju SehrawatNo ratings yet
- Carbonyl Compounds and Carboxylic Acid - Med Easy - Yakeen 2.0 2024 (Legend)Document12 pagesCarbonyl Compounds and Carboxylic Acid - Med Easy - Yakeen 2.0 2024 (Legend)agrawaltwinkle2005No ratings yet
- MINDMAP Alkene, Benzene, HaloalkaneDocument3 pagesMINDMAP Alkene, Benzene, HaloalkaneLeow JiashengNo ratings yet
- Alcohol Phenol ND EthersDocument16 pagesAlcohol Phenol ND Ethersbhawnam.1995No ratings yet
- 5 6289838506726392936Document16 pages5 6289838506726392936GtyNo ratings yet
- Organic Chemistry Oxidation ReactionsDocument9 pagesOrganic Chemistry Oxidation Reactionsgamer boomerNo ratings yet
- Chapter 9 Alcohol Reactions: I. Reactions of Alcohols With Acids and BasesDocument9 pagesChapter 9 Alcohol Reactions: I. Reactions of Alcohols With Acids and BasesRoberto SIlvaNo ratings yet
- 102 Lecture Ch13Document36 pages102 Lecture Ch13macybnzNo ratings yet
- Atkins Chapter 5Document34 pagesAtkins Chapter 5Babylen BahalaNo ratings yet
- Alkenes ReactionsDocument64 pagesAlkenes ReactionsKamran JalilNo ratings yet
- Nucleophilic Substitution and Electrophilic Addition ReactionsDocument4 pagesNucleophilic Substitution and Electrophilic Addition Reactionsjohn mNo ratings yet
- Aqa Mechanisms A21Document4 pagesAqa Mechanisms A21Sarah INo ratings yet
- Alkene Preparation ReactionsDocument13 pagesAlkene Preparation ReactionsSaket ModiNo ratings yet
- Reactions of organic compoundsDocument8 pagesReactions of organic compoundsWojtek LinertowiczNo ratings yet
- TutorialDocument27 pagesTutorialSiti NuraqidahNo ratings yet
- Summary of Alkyne Reactions: H H C H H C BR HDocument1 pageSummary of Alkyne Reactions: H H C H H C BR HKamelNo ratings yet
- Alcohols, Phenols and Ethers NotesDocument55 pagesAlcohols, Phenols and Ethers Notessamay gujratiNo ratings yet
- 27 Alcohol Phenol Ether Formula Sheets Getmarks AppDocument15 pages27 Alcohol Phenol Ether Formula Sheets Getmarks AppFLASH FFNo ratings yet
- 1-s2.0-S0196890417309500-Jun Han Biomass Gasification 2017Document24 pages1-s2.0-S0196890417309500-Jun Han Biomass Gasification 2017nahomNo ratings yet
- (Eduwaves360) OC Hydrocarbon EDocument100 pages(Eduwaves360) OC Hydrocarbon Ekrutika goharkar100% (1)
- Nucleus OC Hydrocarbon EDocument94 pagesNucleus OC Hydrocarbon Epoonam sharmaNo ratings yet
- DR Rafiq Zakaria Campus, Maulana Azad College, DR Ahmad Zaheer Lecture SeriesDocument97 pagesDR Rafiq Zakaria Campus, Maulana Azad College, DR Ahmad Zaheer Lecture Seriesmohsin abdul azizNo ratings yet
- OrgChem Quest2 MRII (Bungay)Document3 pagesOrgChem Quest2 MRII (Bungay)Haidee Ramos EdaNo ratings yet
- Homolytic: Click A Box Below To Go To The MechanismDocument29 pagesHomolytic: Click A Box Below To Go To The Mechanismhknhat100% (1)
- Synthesis of testosterone and other steroids from cholesterolDocument7 pagesSynthesis of testosterone and other steroids from cholesterolVishva AegonNo ratings yet
- Organic Chemistry Reacions SummaryDocument22 pagesOrganic Chemistry Reacions SummaryvgettinfatNo ratings yet
- 12a Alkena Dan Reaksinya Bagian 2Document91 pages12a Alkena Dan Reaksinya Bagian 2ElisNo ratings yet
- Organic Name ReactionsDocument2 pagesOrganic Name ReactionsPratham ZalaNo ratings yet
- AlkenesDocument9 pagesAlkenesNIHAAL KANDPALNo ratings yet
- Chemistry Test-Ii: Part-I Section-I Single Correct Choice Type 1. (D)Document19 pagesChemistry Test-Ii: Part-I Section-I Single Correct Choice Type 1. (D)aayushNo ratings yet
- Bansal Classes Organic Part 2Document195 pagesBansal Classes Organic Part 2Brain MasterNo ratings yet
- Chapter 9 Alcohol Reactions: I. Reactions of Alcohols With Acids and BasesDocument9 pagesChapter 9 Alcohol Reactions: I. Reactions of Alcohols With Acids and BasesRoberto SIlvaNo ratings yet
- Reaction of Ketone CompleteDocument1 pageReaction of Ketone CompleteJoko SusiloNo ratings yet
- Theory Notes On Carboxylic Acid & Its DerivativesDocument15 pagesTheory Notes On Carboxylic Acid & Its Derivativeshemachaturvedi560No ratings yet
- Nucleophilic Reactions Involving Enolate AnionsDocument44 pagesNucleophilic Reactions Involving Enolate AnionsRia SafitriNo ratings yet
- Name Reaction by BP SirDocument35 pagesName Reaction by BP Siraadishuklaa2006No ratings yet
- Chapter 10 - Propylene Derivatives PDFDocument5 pagesChapter 10 - Propylene Derivatives PDFAlejandro Estrella GutiérrezNo ratings yet
- Frequency FactorDocument32 pagesFrequency FactorDHEERESH RAJPUTNo ratings yet
- Alkyl Halides & Aryl Halides-02 - Solved ProblemsDocument13 pagesAlkyl Halides & Aryl Halides-02 - Solved ProblemsRaju SinghNo ratings yet
- Annual Reports in Organic Synthesis — 1971From EverandAnnual Reports in Organic Synthesis — 1971John McMurryNo ratings yet
- KIMIA K1 Trial 2008Document26 pagesKIMIA K1 Trial 2008Naguib ZakariaNo ratings yet
- Alkane AlkenepropertiesDocument3 pagesAlkane AlkenepropertiesNaguib Zakaria100% (2)
- How To Solve Electrochemistry ProblemDocument1 pageHow To Solve Electrochemistry ProblemNaguib Zakaria67% (3)
- Peka Form 5 2 (Exp No 1.3)Document3 pagesPeka Form 5 2 (Exp No 1.3)Naguib Zakaria100% (4)
- Emperical Formula and Molecular FormulaDocument4 pagesEmperical Formula and Molecular FormulaNaguib Zakaria100% (1)
- Electrochemistry TestDocument7 pagesElectrochemistry TestNaguib Zakaria100% (1)
- Electrochemistry Revision: SMK Tunku Ampuan Najihah Chemistry Form 4 Monthly Test 3-2010 1 Hour Test Form 4Document7 pagesElectrochemistry Revision: SMK Tunku Ampuan Najihah Chemistry Form 4 Monthly Test 3-2010 1 Hour Test Form 4Naguib ZakariaNo ratings yet
- Periodic Table: Answering GuideDocument1 pagePeriodic Table: Answering GuideNaguib ZakariaNo ratings yet
- Glossary SPM ChemistryDocument6 pagesGlossary SPM ChemistryMus Staqim BesutNo ratings yet
- Peka F5 1Document4 pagesPeka F5 1Naguib Zakaria88% (8)
- Mole ConceptDocument1 pageMole ConceptNaguib Zakaria67% (3)
- Exercise Chap3 Form 4Document1 pageExercise Chap3 Form 4Naguib ZakariaNo ratings yet
- Periodic TableDocument2 pagesPeriodic TableNaguib Zakaria100% (1)
- Rate of Reaction & Hydrocarbon Chemistry Form 5 Monthly Test 2-2010 1 Hour Test Form 5Document6 pagesRate of Reaction & Hydrocarbon Chemistry Form 5 Monthly Test 2-2010 1 Hour Test Form 5Naguib ZakariaNo ratings yet
- Rate of Reaction & Hydrocarbon Chemistry Form 5 Monthly Test 2-2010 1 Hour Test Form 5Document6 pagesRate of Reaction & Hydrocarbon Chemistry Form 5 Monthly Test 2-2010 1 Hour Test Form 5Naguib ZakariaNo ratings yet
- Rate of Reaction SMK Tunku Ampuan NajihahDocument8 pagesRate of Reaction SMK Tunku Ampuan NajihahNaguib ZakariaNo ratings yet
- Test 1 F4Document7 pagesTest 1 F4Naguib ZakariaNo ratings yet
- Rateof Reaction Part 2Document5 pagesRateof Reaction Part 2Naguib ZakariaNo ratings yet
- CHAP8 Manufactured IndustryDocument12 pagesCHAP8 Manufactured IndustryNaguib Zakaria100% (2)
- Matter Part 1Document4 pagesMatter Part 1Naguib ZakariaNo ratings yet
- Matter Part 2Document4 pagesMatter Part 2Naguib ZakariaNo ratings yet
- Chap 8 Part 2Document3 pagesChap 8 Part 2Naguib ZakariaNo ratings yet
- 3.0 POLYMER Learning Outcome Student Ables To State The MeaningDocument2 pages3.0 POLYMER Learning Outcome Student Ables To State The MeaningNaguib Zakaria100% (1)
- Saltpg 1Document1 pageSaltpg 1Naguib ZakariaNo ratings yet
- Chap 8: SaltDocument2 pagesChap 8: SaltNaguib Zakaria100% (1)
- Chap 8 Part 1Document4 pagesChap 8 Part 1Naguib ZakariaNo ratings yet
- Electrochemistry NoteDocument3 pagesElectrochemistry NoteNaguib Zakaria100% (3)
- Sustaining Tanning with Chromium ConservationDocument28 pagesSustaining Tanning with Chromium ConservationJosé Carlos Vilca AlarcónNo ratings yet
- CLP Type Gear Oil For Difficult ConditionsDocument1 pageCLP Type Gear Oil For Difficult ConditionsdungdhtsNo ratings yet
- PET Geogrid SeriesDocument1 pagePET Geogrid Seriessundra0No ratings yet
- ASME BPVC.II.A-2015 SA-387/SA-387M Standard SpecDocument7 pagesASME BPVC.II.A-2015 SA-387/SA-387M Standard SpecPedro Montes Marin100% (1)
- 2.2 Biological Molecules Ans PDFDocument18 pages2.2 Biological Molecules Ans PDFtess_15No ratings yet
- BSMLS I - MT TitrimetryDocument47 pagesBSMLS I - MT TitrimetrynenaNo ratings yet
- Analyte Normal Range Reference GuideDocument2 pagesAnalyte Normal Range Reference GuideRace MendezNo ratings yet
- 15 Seal Materials 363-376Document14 pages15 Seal Materials 363-376RPINILLA (EICO S.A.)No ratings yet
- Anexo I Do Regulamento EU 1831Document26 pagesAnexo I Do Regulamento EU 1831Sistema de Gestão Rio DesertoNo ratings yet
- Ethanol HandbookDocument110 pagesEthanol HandbookFajar Patriayudha100% (3)
- ,the Photochemical Degradation of Riboflavin SMITH THESIS 1963Document105 pages,the Photochemical Degradation of Riboflavin SMITH THESIS 1963ADEEL ARSALANNo ratings yet
- 0654/42/M/J/22 © Ucles 2022Document26 pages0654/42/M/J/22 © Ucles 2022Zenron27No ratings yet
- WP HFU UNIT EN-A4-hr PDFDocument8 pagesWP HFU UNIT EN-A4-hr PDFDiego CastroNo ratings yet
- Stainless Steel 420 Material Chemical CompositionDocument3 pagesStainless Steel 420 Material Chemical Compositionr arumugamNo ratings yet
- Journal of Macromolecular Science, Part C: To Cite This Article: John R. Martin, Julian F. Johnson & Anthony R. CooperDocument145 pagesJournal of Macromolecular Science, Part C: To Cite This Article: John R. Martin, Julian F. Johnson & Anthony R. CooperRicky Iqbal SNo ratings yet
- Determination of Food AcidityDocument24 pagesDetermination of Food AcidityasmeelyaishakNo ratings yet
- Ionic EquilibriumDocument91 pagesIonic EquilibriumGabrielNo ratings yet
- Architectural Coating Product CatalogDocument5 pagesArchitectural Coating Product CatalogTrường NguyenNo ratings yet
- Tig Plasma77019Document16 pagesTig Plasma77019Luiz CabelloNo ratings yet
- LIPIDS BiochemistryDocument52 pagesLIPIDS BiochemistryMark BagamaspadNo ratings yet
- Tds-Duraplate UhsDocument4 pagesTds-Duraplate UhsAlberto Acosta GongoraNo ratings yet
- FREEDONIA - Specialty Surfactants 2006Document8 pagesFREEDONIA - Specialty Surfactants 2006Andrzej SzymańskiNo ratings yet
- Isotech Calibration 1puntos FijosDocument108 pagesIsotech Calibration 1puntos FijosAlexander MartinezNo ratings yet
- USCG Flyer Concept R5Document2 pagesUSCG Flyer Concept R5Somnath BaruaNo ratings yet
- Ionic CompoundsDocument2 pagesIonic CompoundsShenneth De CastroNo ratings yet
- Mackol CAS-100N: INCI Name: Sodium Coco-Sulfate CAS No.: 68955-19-1 EINECS: 273-257-1Document2 pagesMackol CAS-100N: INCI Name: Sodium Coco-Sulfate CAS No.: 68955-19-1 EINECS: 273-257-1Katrina MillerNo ratings yet
- Formulasi Dan Evaluasi Sediaan Tablet Asam Mefenamat Menggunakan Eksipien Croscarmellose Sodium Sebagai Disintegran Dengan Metode Granulasi BasahDocument8 pagesFormulasi Dan Evaluasi Sediaan Tablet Asam Mefenamat Menggunakan Eksipien Croscarmellose Sodium Sebagai Disintegran Dengan Metode Granulasi BasahressyNo ratings yet
- Bachelor Thesis - Ilfa Van Duijvenbode 5532752 PDFDocument24 pagesBachelor Thesis - Ilfa Van Duijvenbode 5532752 PDFPranav MittalNo ratings yet
- Glycolic Acid: Product InformationDocument2 pagesGlycolic Acid: Product InformationRafael Chiu UrrutiaNo ratings yet
- 2016 Water Quality Annual Report Volume1Document71 pages2016 Water Quality Annual Report Volume1mieNo ratings yet