Professional Documents
Culture Documents
ARTICULO Patterson JCE PH PKa Indicadores 10758
Uploaded by
eyderortega1980Original Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
ARTICULO Patterson JCE PH PKa Indicadores 10758
Uploaded by
eyderortega1980Copyright:
Available Formats
In the Laboratory
JChemEd.chem.wisc.edu Vol. 76 No. 3 March 1999 Journal of Chemical Education 395
A Simplified Method for Finding the pK
a
of an AcidBase
Indicator by Spectrophotometry
George S. Patterson*
Suffolk University, 41 Temple Street, Boston, MA 02114
General chemistry textbooks devote much space to the
important concept of equilibrium. To illustrate one aspect
of equilibrium, a new laboratory experiment on the measure-
ment of an equilibrium constant was desired. Ideally, the ex-
periment would
1. result in a reasonably accurate value of the equilibrium
constant,
2. use a small number of soluti ons that are safe to
handleor at least be familiar to studentsand simple
to dispose,
3. useequipment commonly found in a general chemistry
laboratory, and
4. not exceed the skills of a typical general chemistry
student.
A well-known experiment in analytical and physical
chemi stry laboratory courses i s the spectrophotometri c
determination of the pK
a
of an acidbase indicator (19).
Following published procedures, thisexperiment yieldsaccurate
results using equipment found in most general chemistry labs
(pH meters and single-wavelength spectrophotometers, such
as the Spectronic 20). The acidic and basic solutionsgenerated
during the experiment are probably familiar to studentsand can
be disposed by neutralizing them then pouring them down
the drain. In most published procedures, however, several
buffer solutions must be prepared by technicians before the
lab or by students during the lab. Also, the procedures would
be difficult for most general chemistry studentsto complete in
a three-hour laboratory period. We have developed a simpler
method that uses fewer solutions; pK
a
results for this method
using eight common indicators are reported here.
For the simple method outlined here to work well, there
must be only one acid form of the indicator (HIn) and one
base form (In
) in equilibrium:
HIn
H
+
+ In
If we use concentrations rather than activities, the expression
for the equilibrium constant for the reaction is
K
a
=
[H
+
][In
]
HIn
The logarithmic form of the equation is
pK
a
=pH +log
10
HIn
[In
]
(1)
The acid form of the indicator hasa color, such asyellow,
with its corresponding
max
at one wavelength, and the base
form has another color, such as blue, with its corresponding
max
at a different wavelength. If the cell path length is kept
constant and all solutions contain the same total molarity of
indicator, the acidbase ratio at the
max
of either the acid or
base form is given by (3, 10, 11)
HIn
[In
]
=
AA
In
A
HIn
A
(2)
where A isthe absorbance of the solution containing a certain
total concentration of the acidbase mixture, A
I n
i s the
absorbance of the base form at the same concentration, and A
HIn
isthe absorbance of the acid form at the same concentration.
Substituting the expression for the HInIn
ratio from
eq 2 into eq 1,
pK
a
=pH +log
10
AA
In
A
HIn
A
(3)
or
log
10
AA
In
A
HIn
A
=pK
a
pH
(4)
The pK
a
of an indicator can be determined by either of two
equivalent methods, an algebraic method or a graphical
method. In the algebraic method, sets of pH and absorbance
values are substituted into eq 3 and the pK
a
is calculated for
each set. The pK
a
reported is the average of the calculated
pK
a
s. In the graphical method, log
10
[(A A
In
)/(A
HIn
A)]
vs pH from eq 4 is plotted, and pK
a
is obtained as the x-
intercept. The line should have a slope of 1.
Experimenta l Procedure
A 1% soluti on of phenolphthalei n i n i sopropanol,
pHydrion buffer capsules(pH 4, 7, and 10), and 50% sodium
hydroxide solution were purchased from Fisher Scientific Co.
Aqueous solutions containing 0.04% bromocresol green,
0.04% bromocresol purple, 0.04% bromophenol blue, 0.04%
bromothymol blue, 0.10% methyl orange, 0.10% sodium salt
of methyl red, and 0.04% phenol red were obtained from
Aldrich Chemical Co.
The procedure for determining the pK
a
for bromophenol
blue is described in detail. Conditions for determining the
pK
a
values for the other indicators are tabulated.
A bromophenol bluesolution in itsbaseform was prepared
by dissolving 6 drops of the 0.04% dye solution and 2 drops
of 1 M NaOH in 10 mL of distilled water. To obtain an esti-
mate of
max
, the absorbances of the solution were measured
on a Bausch & Lomb Spectronic 20D spectrophotometer at
20-nm intervalsfrom 560 to 640 nm (Table 1). The absorbance
*Email: spatters@acad.suffolk.edu.
In the Laboratory
396 Journal of Chemical Education Vol. 76 No. 3 March 1999 JChemEd.chem.wisc.edu
at 590 nm was measured to determine the value of
max
more
precisely. Conditions for determining
max
for all indicators
are listed in Table 2.
The solution for determining the pK
a
of bromophenol blue
wasprepared by dissolving 5.0 mL of 0.04% bromophenol blue
solution and the contentsof one pH 4 buffer capsule
1
in water
in a 250-mL volumetric flask. Fifty milliliters of the solu-
tion was poured into each of five 100-mL beakers. Using a
pH meter accurate to 0.01 pH unit, two of the solutions were
adjusted to about pH 3.4 and pH 3.7 by dropwise addition of
1 M HCl. Two other solutionswere adjusted to approximately
pH 4.3 and pH 4.6 by dropwise addition of 1 M NaOH.
The solution in the fifth beaker had a pH of about 4.0. The
approximate pH values used for all the indicators are listed
in Table 3. In each case, solutionswere adjusted to pH values
lower than that of the buffer capsule with 1 M HCl and to pH
valueshigher than that of the buffer capsule with 1 M NaOH.
The absorbances of the five bromophenol blue solutions
were measured with a Bausch & Lomb Spectronic 20D
spectrophotometer. The pH 3.4 solution was then adjusted
to about pH 2 with two drops of concentrated HCl solution
to produce pure HI n, and the absorbance of the resulting
solution was measured to determine A
HIn
. Similarly, the pH
4.6 solution was adjusted to about pH 12 with two drops of
50% NaOH solution to produce pure In
, and the absorbance
of the resulting solution was measured to determine A
In
. The
results for bromophenol blue are displayed in Table 4. The
x-intercept from the plot of log
10
[(A A
I n
)/(A
HI n
A)] vs
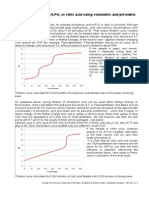
pH (Fig. 1) was3.95, and the slope was0.96. The pK
a
results
for all the indicators investigated are listed in Table 5.
Discussion of Results
The average pK
a
valuesfor the majority of the indicators
obtained using eq 3 have small standard deviations, and the
slopesof the plotsof eq 4 for the indicatorsare generally close
to 1. However, the pK
a
valuesof phenolphthalein determined
by both methods show a much greater uncertainty. Phenol-
phthalein is a dibasic indicator, whose pK
a
valuesare so similar
that the spectrophotometric method doesnot produce accurate
results (15). Methyl red is also a dibasic indicator with a small
difference between pK
a
values(16), but the standard deviation
of the average pK
a
value using eq 3 and the slope of the line
from eq 4 have only slightly larger deviationsthan most of the
other indicators. Other investigators have also found that
methyl red produces reasonably accurate results (1,
7,
8).
The pK
a
valuesdetermined by the algebraic method, using
eq 3, and the graphical method, using eq 4, are essentially
the same, even for phenolphthalein. Most of these values
are within about 0.1 pH unit of the pK
a
values found in the
s e u l a V e c n a b r o s b A . 1 e l b a T
e u l B l o n e h p o m o r B r o f
m n / h t g n e l e v a W e c n a b r o s b A
0 6 5 3 5 5 . 0
0 8 5 2 1 8 . 0
0 0 6 6 9 7 . 0
0 2 6 1 8 2 . 0
0 4 6 7 7 0 . 0
0 9 5 4 1 9 . 0
g n i n i a t b O r o f s n o i t i d n o C . 2 e l b a T
x a m
s r o t a c i d n I r o f
r o t a c i d n I
s p o r D f o . o N m n / h t g n e l e v a W
e y D
n l o S
e s a B
d i c A r o
e g n a R
x a m
( t i L 2 1 )
x a m
l t p x E
n e e r g l o s e r c o m o r B 9 2
a
0 6 6 0 8 5 7 1 6 5 1 6
e l p r u p l o s e r c o m o r B 9 2
a
0 2 6 0 4 5 1 9 5 0 9 5
e u l b l o n e h p o m o r B 6 2
a
0 4 6 0 6 5 2 9 5 0 9 5
e u l b l o m y h t o m o r B 9 2
a
0 6 6 0 8 5 7 1 6 5 1 6
e g n a r o l y h t e M 1 2
b
0 4 5 0 6 4 2 2 5 0 1 5 5 0 5
d e r l y h t e M 1 2
b
0 6 5 0 8 4 0 3 5 5 2 5 0 2 5
n i e l a h t h p l o n e h P 1 2
a
0 8 5 0 0 5 3 5 5 0 5 5
d e r l o n e h P 4 2
a
0 0 6 0 2 5 8 5 5 0 6 5
NOTE: For sulphonphthalein indicators such as bromocresol green and
bromothymol blue and for phenolphthalein, the best choice of wave-
length is the
max
of the base formof the indicator because the base form
has a higher absorbance at its
max
than the acid formhas at its
max
.
Also, the acid formgenerally absorbs very little at the
max
of the base,
whereas the base formabsorbs significantly at the
max
of the acid. The
azo dyes methyl orange and methyl red have the opposite behavior, and
the best wavelength for measuring their pK
a
is the
max
of the acid form.
a
1 M NaOH;
b
1 M HCl.
p e r u s a e M o T d e s U s n o i t u l o S . 3 e l b a T K
a
s r o t a c i d n I f o
r o t a c i d n I
f o l o V
/ r o t a c i d n I
L m
a
r e f f u B
e l u s p a C
) H p (
s e u l a V H p e t a m i x o r p p A
n e e r g l o s e r c o m o r B 0 . 9 4 , 0 . 4 , 3 . 4 , 6 . 4 , 9 . 4 2 . 5
e l p r u p l o s e r c o m o r B 0 . 7 7 , 4 . 5 , 7 . 5 , 0 . 6 , 3 . 6 6 . 6
e u l b l o n e h p o m o r B 0 . 5 4 , 4 . 3 , 7 . 3 , 0 . 4 , 3 . 4 6 . 4
e u l b l o m y h t o m o r B 0 . 9 7 , 4 . 6 , 7 . 6 , 0 . 7 , 3 . 7 6 . 7
e g n a r o l y h t e M 5 . 1 4 , 1 . 3 , 4 . 3 , 7 . 3 , 0 . 4 3 . 4
d e r l y h t e M
b
5 . 1 4 , 4 . 4 , 7 . 4 , 0 . 5 , 3 . 5 6 . 5
n i e l a h t h p l o n e h P 2 . 0 0 1
c
, 8 . 8 , 1 . 9 , 4 . 9 , 7 . 9 0 . 0 1
d e r l o n e h P 0 . 3 7 , 2 . 7 , 5 . 7 , 8 . 7 , 1 . 8 4 . 8
a
Volumes of indicator solutions were chosen such that the highest
absorbance value for each indicator was between 0.7 and 1.0. Results
were not as satisfactory when the highest absorbance value was sig-
nificantly below or above this range.
b
Some methyl red precipitated fromsolution. The solution was filtered
before use.
c
2.0 g of glycerin was added to prevent borax in the buffer from
causing the color to fade.
p . 4 e l b a T K
a
e u l B l o n e h p o m o r B r o f n o i t a n i m r e t e D
H p e c n a b r o s b A pK
a
3 q e m o r f
5 3 . 3 0 7 1 . 0 0 6 . 0 5 9 . 3
5 6 . 3 7 8 2 . 0 8 2 . 0 3 9 . 3
4 9 . 3 1 1 4 . 0 0 0 . 0 4 9 . 3
0 3 . 4 2 6 5 . 0 4 3 . 0 6 9 . 3
4 6 . 4 0 7 6 . 0 5 6 . 0 9 9 . 3
v a 5 9 . 3 2 0 . 0
2 1 A
n I
8 1 8 . 0
2 A
n I H
6 0 0 . 0
log
10
A A
In
A
HIn
A
In the Laboratory
JChemEd.chem.wisc.edu Vol. 76 No. 3 March 1999 Journal of Chemical Education 397
literature at the same ionic strength. The greatest difference
was found for the pK
a
of phenolphthalein, which was about
0.30.4 pH unit lower than the literature value.
This procedure uses fewer solutions than other published
methodsfor finding the pK
a
of an indicator. Solutionsrequired
prior to the lab can be prepared easily or purchased inexpen-
sively. Students in the lab prepare just two solutions, the one
used to determine the
max
of the acid or base form of the
indicator and the stock solution used to measure the pK
a
of
the indicator. The strong acids and bases used are somewhat
dangerousto handle, but studentswould probably be familiar
with their use from previous experiments. At the end of
the experiment, the students themselves or technicians can
neutralize the solutions generated during the experiment and
pour them down the drain.
Conclusions
Thisprocedure for the spectrophotometric determination
of pK
a
values of indicators is a good general chemistry lab
experiment. It leads to accurate results using Spectronic 20s
and pH meters found in most general chemistry labs. The
lab procedure can conveniently be completed within three
hours, because there are few solutions to prepare and other
manipulations are kept to a minimum. In addition, students
are probably familiar with the types of reagents used and
could even dispose their own waste solutions at the end of
the lab period.
N ote
1. The contentsof a pH 4 buffer capsule can be replaced by 1.0 g
of potassium acid phthalate. A mixture of 0.37 g KH
2
PO
4
and 0.60 g
anhydrous Na
2
HPO
4
can be substituted for the contents of a pH 7
buffer capsule.
Litera ture Cited
1. Brown, W. E.; Campbell, J. A. J. Chem. Educ. 1968, 45, 674
675. Several indicatorswere studied by the methodsof Ramette
(4) and Tobey (7).
2. Lai, S. T. F.; Burkhart, R. D. J. Chem. Educ. 1976, 53, 500. The
authorsassume that, for a number of indicators, the absorbances
of HIn at the
max
of In
and In
at the
max
of HIn arenegligible.
Several solutionsof an indicator are prepared with different pH
values, and absorbancesof thesolutionsat each
max
aremeasured.
The pK
a
of the indicator isobtained from the equation pK = pH +
log(A
I n
A
HIn
/A
HIn
A
In
), whereA
HI n
isthe absorbance of a solu-
tion at the
max
of HIn, A
I n
istheabsorbanceof thesolution at the
max
of In
, A
In
istherangeof A
In
values, and A
HIn
istherange
of A
HIn
values. (Notethat thisisthecorrect equation. Theequation
given in ref 2isincorrect.) Data arepresented for thymol blue.
3. Ramette, R. W. Chemical Equilibriumand Analysis; Addison-
Wesley: Reading, MA, 1981; pp 676681. In a general procedure,
studentsmeasuretheabsorption spectra of two solutionscontaining
the acidic and basic forms of an indicator to determine its ana-
lytical wavelength,
max
, or theinstructor tellsthem
max
. Theab-
sorbancesof theacid and baseformsof theindicator at
max
, A
HIn
and A
In
, respectively, are used in eq 2 to calculate [HIn]/[In
].
Three solutions of the indicator with different pHs around its
pK
a
value are prepared by studentsusing an acidconjugate base
buffer, where the pK
a
of the acid isnear the pK
a
of the indicator.
They measure the absorbances and calculate the ionic strengths
of these solutions. Everyone determines the pK
a
value for each
buffered solution using concentration and absorbance data and
activity coefficients, then averagesthe values.
4. Ramette, R. W. J. Chem. Educ. 1963, 40, 252254. Each student is
assigned a different ionic strength at which to prepare solutions
of bromcresol green at different pHs to determine the concen-
tration quotient, Q, of the indicator. First, he or she prepares a
solution of the indicator in sodium acetate solution, using KCl
to achievetheionic strength, and measurestheabsorption spectrum
of the solution. The student then addsseveral aliquotsof acetic
acid solution and finally an aliquot of hydrochloric acid solution
to theindicator solution. After each aliquot isadded, theabsorbance
l
o
g
1
0
[
(
A
A
I
n
-
)
/
(
A
H
I
n
A
)
]
0.800
0.600
0.400
0.200
0.000
0.200
0.400
0.600
0.800
3.50 4.00 4.50 5.00
pH
Figure 1. Plot of log
10
[(A A
In
)/ (A
HIn
A)] vs pH for bromo-
phenol blue.
p f o t n e m e r u s a e M f o s t l u s e R . 5 e l b a T K
a
s r o t a c i d n I f o s e u l a V
r o t a c i d n I
( e u l a V t i L 3 1 p f o ) K
a
h t g n e r t S c i n o I t a
h t g n e r t S c i n o I
d e s U e g n a R
pK
a
3 q E m o r f
pK
a
m o r f
4 q E
f o e p o l S
t o l P 4 q E
1 0 . 0 5 0 . 0 0 1 . 0
n e e r g l o s e r c o m o r B 0 8 . 4 0 7 . 4 6 6 . 4 4 0 . 0 2 0 . 0 2 6 . 4 2 0 . 0 2 6 . 4 4 0 . 1
e l p r u p l o s e r c o m o r B 8 2 . 6 1 2 . 6 2 1 . 6 5 0 . 0 2 0 . 0 8 1 . 6 3 0 . 0 9 1 . 6 4 9 . 0
e u l b l o n e h p o m o r B 6 0 . 4 0 0 . 4 5 8 . 3 3 0 . 0 2 0 . 0 5 9 . 3 2 0 . 0 5 9 . 3 6 9 . 0
e u l b l o m y h t o m o r B 9 1 . 7 3 1 . 7 0 1 . 7 8 0 . 0 4 0 . 0 0 0 . 7 2 0 . 0 0 0 . 7 0 0 . 1
e g n a r o l y h t e M 6 4 . 3 6 4 . 3 6 4 . 3 2 0 . 0 2 4 . 3 2 0 . 0 3 4 . 3 4 0 . 1
d e r l y h t e M 0 0 . 5 0 0 . 5 0 0 . 5 6 0 . 0 3 0 . 0 1 9 . 4 5 0 . 0 0 9 . 4 1 9 . 0
n i e l a h t h p l o n e h P 7 . 9
a
1 . 0 ~ 4 3 . 9 0 2 . 0 7 3 . 9 6 3 . 1
d e r l o n e h P 2 9 . 7 4 8 . 7 1 8 . 7 9 0 . 0 7 0 . 0 5 6 . 7 2 0 . 0 6 6 . 7 3 0 . 1
NOTE: The volumes of acid and base solutions added to the buffered indicator solutions were small
compared to that of the indicator solution itself. Calculations do not need to take this dilution into account,
because it does not affect the values of the pK
a
.
a
Reference 14.
In the Laboratory
398 Journal of Chemical Education Vol. 76 No. 3 March 1999 JChemEd.chem.wisc.edu
of theresulting solution ismeasured at
max
, except that theentire
spectra of the solutions containing 1:1 acetate:acetic acid and
hydrochloric acid are recorded. Absorption readingsare corrected
for dilution. Each student calculates the hydrogen ion concentra-
tion of each solution using the dissociation quotient of acetic acid
at hisor her assigned ionic strength. They calculatepQ valuesfor
each solution using eq 3, then determinean averagevalue. Theclass
poolstheir averagepQ valuesat different ionic strengths, then each
person plotspQ vslog f, using the equation pQ = pK
a
+ log f. In
the equation, f is the ratio of activity coefficients and K
a
is the
acid dissociation constant for the indicator using activities. The
y-intercept of the graph ispK
a
.
5. Salzberg, H. W.; Morrow, J. I.; Cohen, S. R.; Green, M. E. Physical
ChemistryLaboratory: Principlesand Experiments; Macmillan: New
York, 1978; pp 402405. Studentsmeasuretheabsorption spectra
of two solutionscontaining the acidic and basic formsof bromo-
phenol blue to determine its analytical wavelength,
max
. Next,
they dilute solutionscontaining the indicator until one of the so-
lutionshasan absorbance of 0.91.0 at
max
. They further dilute
thissolution to 0.2, 0.4, 0.6, and 0.8 timesitsoriginal strength.
They measure the absorbance of the diluted solutionsat
max
and
preparea Beerslaw plot from theabsorbancesof thefivesolutions.
Each person preparesseveral solutionswith pHsbetween 3.4 and
4.6 that have the same total concentration of bromophenol blue
asthe solution with an absorbance of 0.91.0. The solutionsare
prepared in buffersin thispH range, or thepH isadjusted with hydro-
chloric acid or ammonia solution. Students measure the absor-
banceof thesesolutionsat
max
. They obtain thepK
a
of bromophenol
blue from eq 1 using absorbanciesfrom the Beerslaw data and
the absorbancesof thepH 3.44.6 solutionsor from a plot of eq 3,
similar to the method described here.
6. Sawyer, D. T.; Heineman, W. R.; Beebe, J. M. ChemistryExperiments
for Instrumental Methods; Wiley: New York, 1984; pp 193198.
Studentsmeasuretheabsorption spectra of solutionsof bromothy-
mol blueat pH 1, 7, and 13 and choosetwo wavelengthsto the left
and right of the isosbestic point that have maximum differences
between theabsorbancesof theacid and baseformsof theindicator.
They preparesolutionsof theindicator at seven other pH valuesus-
ing phosphatebuffersand measuretheabsorbancesof thesolutions
at the two wavelengths. For each wavelength, studentsplot A vs
pH using thedata from theninesolutions. ThepK
a
isequal to the
pH at the inflection point of each curve. Also, they determine
the ratiosof [In
2
]/[[HIn
] for each solution from one of the curves
and plot log
10
[In
2
]/[[HIn
] vs pH, using an equation derived
from eq 1. The pK
a
isthe y-intercept of the graph.
7. Tobey, S. W. J. Chem. Educ. 1958, 35, 514515. The absorption
spectra of the acidic and basic forms of methyl red were mea-
sured to determine their respective
max
values. The absorbancies
of both the acidic and basic forms at both
max
values were de-
termined using Beerslaw plots. Four solutionsof methyl red with
the same ionic strength but different pHs were prepared using
the same concentration of sodium acetate and different concen-
trations of acetic acid. The pH of each solution was measured
with a pH meter, and the absorbance was measured at the
max
value of both the acidic and basic forms. Hydrogen ion concen-
trationsfrom pH valuesand concentrationsof the acidic and ba-
sic formsof methyl red in the four solutionsfrom the absorbance
and absorbancy data were used to calculate pK
a
values for each
solution. The valueswere averaged.
8. Walters, D.; Birk, J. P. J. Chem. Educ. 1990, 67, A252A258.
Methyl orange, methyl red, and phenolphthalein were studied. A
number of solutionsof each indicator wereprepared using buffers
that produced a rangeof pH valuessuch that thesolution with low-
est pH contained thepureacid form of theindicator and thesolu-
tion with highest pH contained thepurebaseform. Theabsorbance
of each solution wasmeasured, and plotsof (A
HI n
A
I n
)/(A
A
HI n
)
vs[H
+
]
1
from theequation (A
HIn
A
In
)/(A
A
HI n
) = 1
+K
a
[H
+
]
1
produced straight lineswith slopesequal to K
a
. Thisequation can
be derived from eq 3.
9. Willard, H. H.; Merritt, Jr., L. L.; Dean, J. A. Instrumental Meth-
odsof Analysis, 4th ed; Van Nostrand: New York, 1965; pp 108
109. Studentsobtain the experimental data for bromcresol green
by the method of Ramette (4), except that they measure pH with
a pH meter and everyone usesthe same ionic strength. They de-
termine valuesfor pK
a
by both methodsdescribed by Sawyer et
al. (6).
10. Ramette, R. W. Chemical Equilibriumand Analysis; Addison-
Wesley: Reading, MA, 1981; Chapter 13.
11. Ramette, R. W. J. Chem. Educ. 1967, 44, 647654.
12. LangesHandbook of Chemistry; Dean, J. A., Ed.; McGraw-Hill:
New York, 1992; pp 8.1158.116.
13. Handbook of Analytical Chemistry; Meites, L., Ed.; McGraw-Hill:
New York, 1963; Sec. 3 p 36.
14. Skoog, D. A.; West, D. M. Analytical Chemistry: An Introduction,
4th ed.; Saunders: Philadelphia, 1986; p 164.
15. Kolthoff, I. M. AcidBaseIndicators; Rosenblum, C., Translator;
Macmillan: New York, 1937; pp 112 and 221224.
16. Ramette, R. W.; Dratz, E. A.; Kelly, P. W. J. Phys. Chem. 1962,
66, 527532.
You might also like
- Identifying An Unknown Weak Acids ExperimentDocument18 pagesIdentifying An Unknown Weak Acids ExperimentZati TarhiziNo ratings yet
- Biochem LabDocument10 pagesBiochem LabAlfie16No ratings yet
- Experiment 1 (Introduction)Document16 pagesExperiment 1 (Introduction)Msfaeza HanafiNo ratings yet
- PH MEASUREMENT AND BUFFER PREPARATIONDocument3 pagesPH MEASUREMENT AND BUFFER PREPARATIONJuan Carlos100% (1)
- Lab Report 2Document11 pagesLab Report 2afnan_lion94No ratings yet
- Lab Report 9Document6 pagesLab Report 9api-252715546100% (1)
- pH Titration Lab Experiment Ka DeterminationDocument4 pagespH Titration Lab Experiment Ka DeterminationxmusiqaNo ratings yet
- PH Measurement and Buffer PreparationDocument3 pagesPH Measurement and Buffer Preparationpnduban18No ratings yet
- Titration Curves of Strong and Weak Acids and BasesDocument3 pagesTitration Curves of Strong and Weak Acids and BasesMatthew Runyon50% (2)
- Chem Lab Report 2Document10 pagesChem Lab Report 2api-3105312910% (1)
- Biochemistry LaboratoryDocument13 pagesBiochemistry LaboratoryburgosmjvNo ratings yet
- PH Measurement and Buffer PreparationDocument4 pagesPH Measurement and Buffer PreparationCarmelle Zia ReyesNo ratings yet
- Lab Report 6 Acid and BaseDocument5 pagesLab Report 6 Acid and BasesayaanaNo ratings yet
- AP Chemistry - Titration Curves of Strong and Weak Acids and BasesDocument5 pagesAP Chemistry - Titration Curves of Strong and Weak Acids and BasesJonathan Chen100% (2)
- PH Measurement and Buffer PreparationDocument4 pagesPH Measurement and Buffer PreparationRika MuhiNo ratings yet
- Experiment 4Document5 pagesExperiment 4Ian Joseph Velasco BraganciaNo ratings yet
- Lab Report 4Document22 pagesLab Report 4wilhelminaanimNo ratings yet
- PKa Lab Report 3Document14 pagesPKa Lab Report 3Amanda Wang100% (2)
- Atq E4Document3 pagesAtq E4BuiHopeNo ratings yet
- Experiment 4 PDFDocument7 pagesExperiment 4 PDFsaiNo ratings yet
- pH Titration Soft DrinksDocument7 pagespH Titration Soft DrinksLakshmankumar TjpsNo ratings yet
- Chem 18.1 Experiment 6 Formal ReportDocument5 pagesChem 18.1 Experiment 6 Formal Reportlouize_1496No ratings yet
- Determination of pKa for Weak AcidDocument5 pagesDetermination of pKa for Weak AcidSonu DubeyNo ratings yet
- Experiment 3 Pre-LabDocument3 pagesExperiment 3 Pre-LabKristen LivingstonNo ratings yet
- 11 7 Ka1 For H3PO4 Titrn PDFDocument6 pages11 7 Ka1 For H3PO4 Titrn PDFGaotingweNo ratings yet
- Determining the pKa of Bromothymol BlueDocument6 pagesDetermining the pKa of Bromothymol BlueBriana HalbertNo ratings yet
- Titration Lab ReportDocument13 pagesTitration Lab Reportapi-341133750No ratings yet
- Buffer SolutionDocument24 pagesBuffer SolutionpumeanandaNo ratings yet
- SPECTROPHOTOMETRIC pKa DETERMINATIONDocument10 pagesSPECTROPHOTOMETRIC pKa DETERMINATIONjoanne_blanco100% (1)
- Ka Detn SpectrophDocument3 pagesKa Detn SpectropheveltoncNo ratings yet
- 5: PH Measurement and Its Applications (Experiment) : ObjectivesDocument19 pages5: PH Measurement and Its Applications (Experiment) : ObjectivesNajmi NasirNo ratings yet
- HPLC Analysis of AspirinDocument4 pagesHPLC Analysis of AspirinRazel Elaine Grace CataluñaNo ratings yet
- PH Meter Universal IndicatorDocument4 pagesPH Meter Universal IndicatorNeeta PandeyNo ratings yet
- Designing Acid-Base Titrations GuideDocument12 pagesDesigning Acid-Base Titrations GuideNeen NaazNo ratings yet
- PH and BuffersDocument4 pagesPH and BuffersNirmalya Chatterjee100% (1)
- Determination of pKa of Bromothymol BlueDocument9 pagesDetermination of pKa of Bromothymol BlueLuqman HakimNo ratings yet
- Factors Influenc, Ing The Reduction of Alkaline Copper Reagents by GlucoseDocument18 pagesFactors Influenc, Ing The Reduction of Alkaline Copper Reagents by GlucoseNurul Aulia HusainNo ratings yet
- Chem Lab-3Document15 pagesChem Lab-3api-389948390No ratings yet
- YesDocument4 pagesYesaccel.cyclone.099No ratings yet
- Identifying An Unknown Weak Acids ExperimentDocument18 pagesIdentifying An Unknown Weak Acids Experimentgeek3112100% (5)
- Laboratory Activity No 3Document6 pagesLaboratory Activity No 3Bok MatthewNo ratings yet
- PH Measurement and Buffer PreparationDocument6 pagesPH Measurement and Buffer PreparationJamesMartinDavidNo ratings yet
- PH Measurement and Buffer Preparation (Formal Report)Document5 pagesPH Measurement and Buffer Preparation (Formal Report)Paul Benjomin Agregado50% (4)
- Selecting Buffer pH in RP-HPLC for Optimal Analyte SeparationDocument1 pageSelecting Buffer pH in RP-HPLC for Optimal Analyte SeparationCristian MeneguzziNo ratings yet
- Chem 131A: PH and Buffers ExperimentDocument4 pagesChem 131A: PH and Buffers ExperimentLinh NguyễnNo ratings yet
- BIOCHEM EXP 1 PPT Final 2 PDFDocument8 pagesBIOCHEM EXP 1 PPT Final 2 PDFEUNICE FIGUEROANo ratings yet
- Determination of H PO or Citric Acid Using Volumetric and Ph-Metric TitrationsDocument2 pagesDetermination of H PO or Citric Acid Using Volumetric and Ph-Metric TitrationsMayfurNo ratings yet
- Expt.1 BiochemDocument4 pagesExpt.1 BiochemMc de RamosNo ratings yet
- Lab ReportDocument10 pagesLab Reportapi-327825157No ratings yet
- Chem 160.1 Ex2 BufferDocument8 pagesChem 160.1 Ex2 BufferAsi JenNo ratings yet
- Experiment 11 Results and Discussion Report: Potentiometric Determination of The Purity and Dissociation Constant of Potassium Hydrogen PhthalateDocument4 pagesExperiment 11 Results and Discussion Report: Potentiometric Determination of The Purity and Dissociation Constant of Potassium Hydrogen PhthalateNathalie Dagmang80% (10)
- Phytochemical Analysis Laboratory Manual: Hebron University Prepared by Dr. Abdel Qader A. QawasmehDocument20 pagesPhytochemical Analysis Laboratory Manual: Hebron University Prepared by Dr. Abdel Qader A. QawasmehQOSSAY ALHROUSHNo ratings yet
- New colorimetric method for quantitative phospholipid estimation without acid digestionDocument3 pagesNew colorimetric method for quantitative phospholipid estimation without acid digestionZoya ShaikhNo ratings yet
- Potentiometric Acid-Base Tit RationsDocument2 pagesPotentiometric Acid-Base Tit RationsMark del RosarioNo ratings yet
- Experiment 4 - Potentiometric TitrationDocument11 pagesExperiment 4 - Potentiometric TitrationJoemer Absalon Adorna100% (2)
- Alkalimetry in Alcohol-Water Mixtures With Potentiometric End-Point Detection Critical Remarks On A Newer Method ofDocument6 pagesAlkalimetry in Alcohol-Water Mixtures With Potentiometric End-Point Detection Critical Remarks On A Newer Method ofErik AristyaNo ratings yet
- Determining Ionization Constants of Anti-Inflammatory CompoundsDocument9 pagesDetermining Ionization Constants of Anti-Inflammatory CompoundsAman AmanNo ratings yet
- Half Titration Lab ReportDocument6 pagesHalf Titration Lab Reportapi-20078641867% (3)
- Thermometric Titrimetry: International Series of Monographs in Analytical ChemistryFrom EverandThermometric Titrimetry: International Series of Monographs in Analytical ChemistryNo ratings yet
- XXIVth International Congress of Pure and Applied Chemistry: Plenary and Main Section Lectures Presented at Hamburg, Federal Republic of Germany, 2–8 September 1973From EverandXXIVth International Congress of Pure and Applied Chemistry: Plenary and Main Section Lectures Presented at Hamburg, Federal Republic of Germany, 2–8 September 1973No ratings yet
- Curso Operador de CaixaDocument55 pagesCurso Operador de CaixaGeorge ZoomNo ratings yet
- The Georgia GuidestonesDocument50 pagesThe Georgia Guidestonesantivenin9No ratings yet
- I Am Sharing 'GP-Tort by Jian en Tan' With YouDocument5 pagesI Am Sharing 'GP-Tort by Jian en Tan' With YouFranklin ClintonNo ratings yet
- NPV & Other Investment Rules - RossDocument30 pagesNPV & Other Investment Rules - RossPrometheus Smith100% (1)
- Legal Realism Study NotesDocument4 pagesLegal Realism Study Noteskapil shrivastavaNo ratings yet
- FORM 1 Chapter 5 Perimeter, Area and Volume 2014Document18 pagesFORM 1 Chapter 5 Perimeter, Area and Volume 2014Amjad RanaNo ratings yet
- 120-122Dr RAJIVARANJANDocument4 pages120-122Dr RAJIVARANJANritz meshNo ratings yet
- The Province of North Cotabato, Et Al - V - The Government of The Republic of The Philippines, Et Al .Document11 pagesThe Province of North Cotabato, Et Al - V - The Government of The Republic of The Philippines, Et Al .Arbee ArquizaNo ratings yet
- A Midsummer Night's DreamDocument24 pagesA Midsummer Night's DreamMaria Iuga100% (1)
- The Title of EKSAKTA Journal TemplateDocument4 pagesThe Title of EKSAKTA Journal Templatemaisari utamiNo ratings yet
- FUJITSU 1.2GHz PLL Frequency Synthesizer Data SheetDocument26 pagesFUJITSU 1.2GHz PLL Frequency Synthesizer Data SheetherzvaiNo ratings yet
- Swami Hariharananda's Vision of Kriya YogaDocument103 pagesSwami Hariharananda's Vision of Kriya YogaOrn-uma DaumNo ratings yet
- A6 at CommandsDocument177 pagesA6 at CommandsAlvaro RamosNo ratings yet
- The Paradigmatic Crisis in Chinese Studies: Paradoxes in Social and Economic HistoryDocument44 pagesThe Paradigmatic Crisis in Chinese Studies: Paradoxes in Social and Economic HistoryYadanarHninNo ratings yet
- Cheese Comes Up Many Times in The Bible LWML DevotionDocument1 pageCheese Comes Up Many Times in The Bible LWML Devotionnathan_yamahaNo ratings yet
- PC1431 MasteringPhysics Assignment 7Document16 pagesPC1431 MasteringPhysics Assignment 7stpmoment0% (4)
- The Relation Between Learning Styles, The Big Five Personality Traits and Achievement MotivationDocument12 pagesThe Relation Between Learning Styles, The Big Five Personality Traits and Achievement MotivationJVolasonNo ratings yet
- Raj Bank PPT 2003Document32 pagesRaj Bank PPT 2003Sunny Bhatt100% (1)
- Chicken Soup With Barley Resource PackDocument24 pagesChicken Soup With Barley Resource PackSreelakshmi ShylajanNo ratings yet
- Application Letter and ResumeDocument2 pagesApplication Letter and ResumeLindon Jay EnclunaNo ratings yet
- Indian Political SystemDocument25 pagesIndian Political SystemAyeza AmerNo ratings yet
- Muslim Women in The Tenement Gardens of Colombo: A Story of Marginalization, Legitimized by A Culture of OppressionDocument36 pagesMuslim Women in The Tenement Gardens of Colombo: A Story of Marginalization, Legitimized by A Culture of OppressionSocial Scientists' AssociationNo ratings yet
- Life of BuddhaDocument53 pagesLife of BuddhaPrashant SrivastavaNo ratings yet
- Evaluate Narrativs Based On How The Author Developed The Element THEMEDocument30 pagesEvaluate Narrativs Based On How The Author Developed The Element THEMEDianArtemiz Mata Valcoba80% (25)
- Question TagsDocument2 pagesQuestion TagsAlex MontalvoNo ratings yet
- Reflection Paper About Locomotor and Non-Locomotor MovementsDocument1 pageReflection Paper About Locomotor and Non-Locomotor MovementsanaryNo ratings yet
- The Twin BlockDocument19 pagesThe Twin BlockMohammed Hossam ElnaggarNo ratings yet
- Flipbook Threshold DE PDFDocument80 pagesFlipbook Threshold DE PDFRegulator0No ratings yet
- English10 q4 w7 Mod7Document16 pagesEnglish10 q4 w7 Mod7Richander M. MocallayNo ratings yet
- Termination and Adoption PetitionDocument4 pagesTermination and Adoption Petitionjuakin12350% (4)