Professional Documents
Culture Documents
Building Thermal Performance and PCM - 1
Uploaded by
Srba IlicOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Building Thermal Performance and PCM - 1
Uploaded by
Srba IlicCopyright:
Available Formats
19/02/2009
1
The University of Nottingham Feb/09 LTR
Building Thermal Performance and
Phase Change Materials
LUCELIA TARANTO RODRIGUES
Cartoons by Weikai Gong
The University of Nottingham Feb/09 LTR
1. PRINCIPLES OF HEAT TRANSFER
19/02/2009
2
The University of Nottingham Feb/09 LTR
Heat Transfer
Transit of energy caused by a temperature difference, always from the
highest to the lowest
It can occur by radiation, convection or conduction
In conduction and convection the energy travels through
matter
In radiation the energy travels in electromagnetic waves
The University of Nottingham Feb/09 LTR
Radiation
Radiation is the transmission of energy through electromagnetic waves
It can happen in empty space, it does not need a matter
However, the emission (generation) of the radiation does require a matter to
happen
Central actor regarding passive design is the SUN
Energy is emitted by the sun and arrives on earth in the form of heat
and light
As it travels in parallel rays, the perpendicular position identifies the
maximum density of rays striking a surface
19/02/2009
3
The University of Nottingham Feb/09 LTR
Reflection, Transmission and Absorption
When the emitted energy hits a
surface it is reflected, transmitted
or absorbed
All surfaces both reflect and
absorb radiation but do so in
different ways
For any transparent or semi-transparent layer, part of the incident
radiation is transmitted, another portion is reflected and the remaining is
absorbed
The University of Nottingham Feb/09 LTR
Fancy names to those properties
Emissivity (): a measure of a materials ability to radiate energy
Absorptivity (): the capacity of a material to absorb incident radiant
energy
Reflectivity (): the capacity of a surface to reflect incident radiant
energy
Transmissivity (): the fraction of incident radiant energy being
transmitted through a transparent object
19/02/2009
4
The University of Nottingham Feb/09 LTR
First Law of Thermodynamics
Law of the conservation of energy
Energy can be transformed but it can neither be created nor destroyed.
Absorptivity ()
Reflectivity ()
Transmissivity ()
1 = + +
The University of Nottingham Feb/09 LTR
Conduction
In wall
19/02/2009
5
The University of Nottingham Feb/09 LTR
Conduction
The absorbed portion of radiant energy (like solar radiation) is
transformed to another form of energy (like heat energy)
The heat energy is transported between parts of the material by transfer
of kinetic energy between particles
The stimulated molecules impact the adjacent molecules with their
vibration dissipating and spreading the energy
Absorbed radiation is redistributed through the material by
conduction
Phenomenon of maintenance of balance: always in the direction
of the decreasing temperature
The University of Nottingham Feb/09 LTR
Convection
In wall
19/02/2009
6
The University of Nottingham Feb/09 LTR
Convection
Is the heat transfer due to the actual motion of the fluid itself
When the heated molecules move away from the source they are replaced
by new unheated molecules
To transfer the heat from a solid material to a fluid (liquid or air) it is
necessary to make it move across a radiant surface to agitate the
molecules
The heat transfer is radiation
The fluid movement is convection
Can be forced by a machine (fan or pump)
Or natural as the vibration of the molecules makes them less
dense and so lighter to rise
The University of Nottingham Feb/09 LTR
Summary of Heat Transfer Mechanism
19/02/2009
7
The University of Nottingham Feb/09 LTR
2. THERMOPHYSICAL PROPERTIES OF MATERIALS
The University of Nottingham Feb/09 LTR
Thermophysical Properties of Materials
The rate of conduction dependent on the density, capacity of receiving
and capacity of passing it on:
Density (): the mass of a material that fills a unit volume (kg/m
3
)
Specific heat (c): the quantity of energy needed to produce a
temperature change in a mass of material (J/kg
o
C)
Thermal conductivity (k): the ability of a material to conduct heat at a
unit thickness of material with both surfaces at a unit temperature
difference (W/m
o
C)
Opposite of Thermal resistivity (m
o
C/W)
k
r
1
=
19/02/2009
8
The University of Nottingham Feb/09 LTR
Some examples
Conductivity
(W/m
o
C)
Density
(kg/m
3
)
Specific Heat
(J/kg
o
C)
Steel 45 7800 480
PVC (regular) 0.16 1380 1000
Earth (common) 1.28 1460 880
Expanded polystyrene 0.035 23 1470
Cement (regular) 0.72 1860 840
Cement screed 1.4 2100 650
Plasterboard 0.17 800 1090
Heavyweight concrete 1.3 2000 840
Medium weight concrete block 0.86 1970 840
Lightweight concrete 0.2 620 840
Aerated brick 0.3 1000 840
Inner leaf brick 0.62 1800 840
Reinforced brick 1.1 1920 840
Outer leaf brick 0.96 2000 650
Ceramic tiles 1.20 2000 850
Softwood 0.17 550 1880
Hardwood 0.05 90 2810
The University of Nottingham Feb/09 LTR
Worth Mentioning:
These properties are time-dependent because of material temperature
and/or moisture fluctuations
May also be direction and/or position dependent if the material is non-
homogeneous or anisotropic
Calculations might assume these properties are constant
19/02/2009
9
The University of Nottingham Feb/09 LTR
Can you fire walk?
Wood is a poor conductor (isnt it used for handles
of cookware?)
Although its temperature is high, relatively little
heat is conducted to the feet
The University of Nottingham Feb/09 LTR
What is insulation?
Is a material with low absorption, low
conductivity and trapped air like a
jumper
Air is a terrible conductor of heat
It can however transport heat
effectively by convection
The solution is to trap air in
countless tiny fibers so convection is
inhibited and the air becomes a
great insulator
19/02/2009
10
The University of Nottingham Feb/09 LTR
Thermal Effusivity
Thermal Effusivity () of a material is often referred to as Thermal Inertia
Determines the interfacial temperature when two objects at different
temperatures touch
It is the square roots of conductivity, density and heat capacity
A measure of the materials capacity to exchange thermal
energy with its surroundings
High effusivity means that a material will more readily absorb a heat
flux on a surface
The University of Nottingham Feb/09 LTR
Thermal Effusivity
The effusivity of the skin and the object it touches determines the
interfacial surface temperatures of it
If the effusivity of a material is high (i.e. ceramic or metal) the
interfacial temperature is lower than if the effusivity is low (i.e. Wood
or carpet)
19/02/2009
11
The University of Nottingham Feb/09 LTR
3. CONDUCTION AND STORAGE OF HEAT
The University of Nottingham Feb/09 LTR
Quantifying Heat Conduction
Fourier's law governs heat conduction
Rate of heat flow through a homogenous material is proportional to
the area of the section at right angles to the direction of heat flow and
to the temperature difference along the path of heat flow
x
T
k q
=
q = heat flux (W/m)
k = conductivity (W/mC)
T/x = temperature gradient (C/m)
19/02/2009
12
The University of Nottingham Feb/09 LTR
Fouriers Law
L
T T
k q
2 1
=
q = heat flux (W/m)
k = conductivity (W/m
o
C)
T1 = initial temperature (
o
C)
T2 = final temperature (
o
C)
L = thickness (m)
The University of Nottingham Feb/09 LTR
Conductance or U-Value
R L
k
U
1
= =
U= conductance (W/m
o
C)
R= resistance (m
o
C/W)
k= conductivity (W/m
o
C)
L= thickness (m)
Therefore, to predict the thermal behaviour of a building, it is necessary to
take into account the thickness L
Conductance is the relation between the material conductivity and its
thickness
Also referred to as the U-Value or Thermal Transmittance
It is the converse of R-Value or Resistance
19/02/2009
13
The University of Nottingham Feb/09 LTR
Conductance or U-Value
Rn R R
U
+ + +
=
... 2 1
1
Convenient for multi layered walls
U= conductance (W/m
o
C)
R= resistance (m
o
C/W)
3 3 2 2 1 1
1
/k L /k L /k L
U
+ +
=
13 . 0 6 14 . 0
1
+ +
= U
U= 0.16 W/m
o
C
R= 6.22 m
o
C/W
The University of Nottingham Feb/09 LTR
Also Worth Mentioning
Two walls with the same U-Value will conduct the same amount of heat
even though the thicknesses and materials may differ
Two walls of the same materials but different thicknesses will have
different U-Values
Ahhh..!
19/02/2009
14
The University of Nottingham Feb/09 LTR
In out
Thermal Storage in Thermal Mass
The University of Nottingham Feb/09 LTR
Thermal Storage in Thermal Mass
in out
In thermal mass the heat transfer process occurs
in 4 steps:
Heat is radiated from a body (i.e. sun) to the
surface of the material where it is absorbed
Heat is conducted from the warmer surface
to the cooler interior of the material
When the surface becomes warmer than its
surroundings it radiates heat back to it
becoming cooler again
Heat from the warmer interior is conducted
back to the surface
19/02/2009
15
The University of Nottingham Feb/09 LTR
Thermal Mass
Hence for a material to have good thermal mass it has to have a
combination of factors
Has to be able to conduct heat but not too fast so it wont escape:
moderate conductivity
Should be able to store heat: high heat capacity and density
Should be able to absorb solar radiation and to radiate heat back to the
space: good surface absorption and high emissivity
Admittance factor (Y) takes account of those factors and relates to
the heat that enters the wall from the space and then return to the
space (W/m
o
C)
The University of Nottingham Feb/09 LTR
Time lag and Decrement Factor
Time lag is the time delay on the heat conduction caused by the materials
mass
Decrement factor is the reduction in cyclical temperature on the inside
surface compared to the outside surface
19/02/2009
16
The University of Nottingham Feb/09 LTR
4. PHASE CHANGE MATERIAL (PCM)
The University of Nottingham Feb/09 LTR
Sensible Heat VS Latent Heat
Sensible heat is the one we can feel
Latent heat is the hidden heat
Thermal mass stores sensible heat most common way of heat storage in
buildings
Latent heat provides much higher storage capacity at a smaller temperature
difference
Latent heat is the amount of energy in the form of heat released or
absorbed by a substance during a change of state
Phase Change Materials (PCM) are substances capable of changing state
19/02/2009
17
The University of Nottingham Feb/09 LTR
Sensible Heat VS Latent Heat
Why you put ice cubes in your drink? Because water is a PCM!
As they melt they absorb heat from their environment; as they solidify
in the freezer compartment, they release heat into it
The University of Nottingham Feb/09 LTR
Phase Change Materials: Water
To change its state water, like other substances, uses heat
energy
The heat breaks the waters hydrogen bonds
During the change of state the substances temperature
stays constant
19/02/2009
18
The University of Nottingham Feb/09 LTR
Properties of Water
Specific heat: quantity of energy needed to produce a temperature change
in a mass of material (J/kg
o
C)
Specific heat capacity water at 25
o
C approx 4.18 kJ/kg
o
C
Specific heat capacity ice at -10
o
C approx 2.05 kJ/kg
o
C
Specific heat capacity vapour at 100
o
C approx 2.08 kJ/kg
o
C
Specific latent heat: the quantity of heat energy required to change the state
of a unit mass of a substance (J/kg)
Latent heat of fusion approx 334 kJ/kg
Latent heat of evaporation approx 2260 kJ/kg
The University of Nottingham Feb/09 LTR
Properties of Water
Unfortunately, for various reasons, water cannot be used as a PCM in
building applications...
19/02/2009
19
The University of Nottingham Feb/09 LTR
Desirable Properties for PCM in Building
Applications
Thermophysical
Melting/ solidifying temperature in the desired range
High latent heat per unit volume so less volume is required
High thermal conductivity of all phases to assist the charging and discharging
of energy of the system
Small volume change and small vapor pressure to reduce containment
problems
Congruent change of phase for a constant storage capacity of the material
with each melting/ solidifying cycle
High specific heat to provide additional significant sensible heat storage
The University of Nottingham Feb/09 LTR
Desirable Properties for PCM in Building
Applications
Chemical
Complete reversible melting/ solidifying cycle
No degradation after a large number of melting/ solidifying cycle
No corrosiveness to the construction materials
Non-toxic, non-flammable and non-explosive material for safety
Kinetic
High nucleation rate to avoid super cooling of the liquid phase
High rate of crystal growth to meet demand of heat recovery from the system
19/02/2009
20
The University of Nottingham Feb/09 LTR
How to overcome the problems?
Direct immersion method: the PCM in liquid phase might flow away
Macroencapsulation: containments usually larger than 1 cm in diameter,
most common form of encapsulation
Microencapsulation: containments smaller than 1 mm in diameter,
recently developed new form of encapsulation
The University of Nottingham Feb/09 LTR
Macroencapsulated PCM
19/02/2009
21
The University of Nottingham Feb/09 LTR
Microencapsulated PCM
The University of Nottingham Feb/09 LTR
Types of PCM for Building Applications
Organic compounds: Paraffin and Fatty acids
Chemically stable, non-corrosive, high latent heat and low vapour
pressure
Flammable, high changes in volume and low conductivity
Inorganic compounds: Salt hydrates
Higher latent heat per unit volume, high thermal conductivity, lower in
cost and non-flammable
Corrosive to most metals, supercooling problems and decomposition
can affect their phase change properties
19/02/2009
22
The University of Nottingham Feb/09 LTR
Application areas for PCMs in buildings
1. Latent heat store for space
heating
2. Plaster and compound systems
with high heat storage capacity
3. Transparent insulation and
daylighting schemes
4. Shading PCM compounding
system
5. PCM in gypsum products and
paints
6. PCM to buffer temperature
variations in solar-air-systems
The University of Nottingham Feb/09 LTR
PCMs in the Building Envelope
The three principal PCMs investigated for use in building envelopes are
salt hydrates, fatty acids and paraffins
Sensible heat capacity of the building envelope can be summed to or
substituted by the latent heat capacity
Integrate thermal mass effect without adding weight to the
construction
PCM in the building envelope is an active component diminishing the
temperature swings and contributing to ideal room conditions
PCM wallboards are usually the easiest to install in new as well as retrofit
constructions and can simply replace the existing wallboards
19/02/2009
23
The University of Nottingham Feb/09 LTR
The Effect of Thermal Mass
HEAVY WEIGHT WALL:
OUT IN
Too
cold
Too
hot
The University of Nottingham Feb/09 LTR
OUT IN
Too
cold
Too
hot
The Effect of Thermal Mass
LIGHT WEIGHT WALL:
19/02/2009
24
The University of Nottingham Feb/09 LTR
OUT IN
Too
cold
Too
hot
The Effect of Thermal Mass
LIGHT WEIGHT WALL WITH PCM BOARD:
The University of Nottingham Feb/09 LTR
PCM Quick Summary
It is an active building component
Price should not be compared to innactive materials
It can be applied in buildings in many different ways but the easiest in
passive design is to use PCM in the buildings envelope
PCM Boards are especially practical in lightweight buildings or retrofit
It can efficiently reduce and shift the peak temperature and improve comfort
Savings not just on need for air-conditioning but also on use of off-peak
energy
Good design is crucial as PCM should be combined with effective and
controlable ventilation
19/02/2009
25
The University of Nottingham Feb/09 LTR
5. EXAMPLES
The University of Nottingham Feb/09 LTR
BASF Climate Control Micronal PCM
Knauf PCM Smartboard
3kg of Micronal PCM per m
2
Melting/Solidifying temperatures:
23
o
C or 26
o
C
Heat storage capacity of 110 kJ/kg
So 330kJ/ m
2
www.micronal.de/portal/basf/ien/dt.jsp?setCursor=1_290798
Microencapsulated paraffin wax
19/02/2009
26
The University of Nottingham Feb/09 LTR
Knauf PCM SmartBoard 23 Enthalpy
The University of Nottingham Feb/09 LTR
Knauf PCM SmartBoard 23 Enthalpy
Savings in CO2 emissions of over 100 t per year in this case
19/02/2009
27
The University of Nottingham Feb/09 LTR
DuPont Energain Panels
http://energain.co.uk/Energain/en_GB/index.html
The University of Nottingham Feb/09 LTR
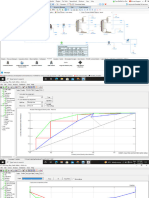
Does it work?
10
12
14
16
18
20
22
24
26
28
30
32
34
36
38
40
42
44
0
.0
0
3
.0
0
6
.0
0
9
.0
0
1
2
.0
0
1
5
. 0
0
1
8
. 0
0
2
1
.0
0
0
.0
0
3
.0
0
6
.0
0
9
.0
0
1
2
. 0
0
1
5
. 0
0
1
8
.0
0
2
1
. 0
0
Time (minute)
T
e
m
p
e
r
a
t
u
r
e
(
o
C
)
External Air Temp No PCM Air Temp PCM Air Temp
19/02/2009
28
The University of Nottingham Feb/09 LTR
Designed by Derek Trowell Architects
The BASF House
The University of Nottingham Feb/09 LTR
Affordability + Energy-efficiency
South and North buffer zones
High insulation U-Values 0.15W/m
2
K
Low-e Double Glazing
Earth-Air Heat Exchanger
Solar collectors and biomass boiler
House intelligence
Controlled natural ventilation
Phase Change Materials
The BASF House
19/02/2009
29
The University of Nottingham Feb/09 LTR
The University of Nottingham Feb/09 LTR
19/02/2009
30
The University of Nottingham Feb/09 LTR
The PCMexpress Simulation Software
Developed in co-operation with the Fraunhofer Institute for Solar Energy
Technology (ISE) in Freiburg, the Valentin Energiesoftware Company and
other BASF industrial partners
Allows for planning and simulation of buildings with phase change
materials
Designed to support architects and planners
Enables secure decision making for the sizing of the overall system
http://www.valentin.de/pcm/forms/form.pcm.php?lang=en
The University of Nottingham Feb/09 LTR
QUESTIONS AND EXERCISE
You might also like
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- 13 Worksheet PKTDocument8 pages13 Worksheet PKTAllyza Alimeos SobosoboNo ratings yet
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Determine Specific Air Capacity Using Air Conditioning UnitDocument13 pagesDetermine Specific Air Capacity Using Air Conditioning UnitTan Xing YanNo ratings yet
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- P R o B L e M S o F P R A C T I C e S O F B A S I C A N D A P P L I e D T H e R M o D y N A M I C S I - C - E N G I N eDocument21 pagesP R o B L e M S o F P R A C T I C e S O F B A S I C A N D A P P L I e D T H e R M o D y N A M I C S I - C - E N G I N eAnonymous ncBe0B9bNo ratings yet
- Production of Dimethyl EtherDocument57 pagesProduction of Dimethyl EtherMuhammad AbubakarNo ratings yet
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- 1 BmeDocument44 pages1 BmepmagrawalNo ratings yet
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- Naoe 2205: Heat Transfer: ConductionDocument22 pagesNaoe 2205: Heat Transfer: ConductionWasi Mahmud SiamNo ratings yet
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Steam GenaratorDocument119 pagesSteam GenaratorRavi balanNo ratings yet
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- SPM Phy Quantity of Heat IDocument13 pagesSPM Phy Quantity of Heat ICHINEMEREM EZEHNo ratings yet
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Fizik Skema Pahang 07Document11 pagesFizik Skema Pahang 07slokkro100% (2)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Thermal Conductivity, Resistance and Specific Heat Capacity of Chemically-Treated, Widely-Used Timber For Building-EnvelopeDocument21 pagesThermal Conductivity, Resistance and Specific Heat Capacity of Chemically-Treated, Widely-Used Timber For Building-EnvelopeAlif Mu'tashimNo ratings yet
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Distortion Control MethodsDocument36 pagesDistortion Control Methodsbmkramesh100% (4)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Cambridge Ordinary LevelDocument16 pagesCambridge Ordinary LevelAayan AhmadNo ratings yet
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- ME140 Cantera Function GuideDocument2 pagesME140 Cantera Function GuideBen WolkNo ratings yet
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- Temp and Heat Chapter 10Document18 pagesTemp and Heat Chapter 10erikaNo ratings yet
- Equations in PhysicsDocument2 pagesEquations in PhysicsDhruti MysoreNo ratings yet
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Thermochemistry IntroDocument24 pagesThermochemistry IntroFilip MarkusNo ratings yet
- Unit 4 Specific Heat Capacity and Latent Heat 1Document6 pagesUnit 4 Specific Heat Capacity and Latent Heat 1M Rheza RizqiaputraNo ratings yet
- Week 18 WW2 CompilationDocument52 pagesWeek 18 WW2 CompilationKenberly DingleNo ratings yet
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- Thermodynamics 1: D. 0.095 Cal/g-deg-CDocument17 pagesThermodynamics 1: D. 0.095 Cal/g-deg-CDon Aries Eidos100% (1)
- Midterm Exam - ThermoDocument1 pageMidterm Exam - ThermoJyonah Beniza0% (1)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- Kittel and Kroemer Thermal PhysicsDocument33 pagesKittel and Kroemer Thermal PhysicsAllen Yu80% (5)
- Energy Performance Assesment of FurnaceDocument16 pagesEnergy Performance Assesment of FurnacePranoy Barua100% (2)
- P103 Temperature, Heat, ExpansionDocument33 pagesP103 Temperature, Heat, ExpansionAnonymous 88kXqKNo ratings yet
- Chapter 3Document18 pagesChapter 3eileenNo ratings yet
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- C3. Laws of ThermodynamicsDocument29 pagesC3. Laws of ThermodynamicsLarry MagallanoNo ratings yet
- Specific Heat Practice Sub WorkDocument2 pagesSpecific Heat Practice Sub Workapi-259781257No ratings yet
- First Law of Thermodynamics: Unit IIDocument24 pagesFirst Law of Thermodynamics: Unit IIfrendNo ratings yet
- Personalized, Comprehensive Course ReviewDocument16 pagesPersonalized, Comprehensive Course ReviewLeyla thrxNo ratings yet
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Physics 2007 EUEEDocument8 pagesPhysics 2007 EUEElikinaw demisieNo ratings yet
- Calc ThermDocument11 pagesCalc ThermMasood Alam FarooquiNo ratings yet
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)