Professional Documents
Culture Documents
Venlafaxine
Uploaded by
MariusNeicuCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Venlafaxine
Uploaded by
MariusNeicuCopyright:
Available Formats
Venlafaxine
1
Venlafaxine
Venlafaxine
Systematic (IUPAC) name
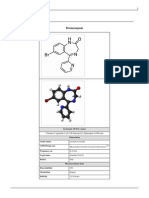
(RS)-1-[2-dimethylamino-1-(4-methoxyphenyl)-ethyl]cyclohexanol
Clinical data
Trade names Efexor, Effexor
AHFS/Drugs.com
monograph
[1]
Licence data
USDailyMed:link
[2]
Pregnancy cat. B2 (AU) C (US)
Legal status Prescription Only (S4) (AU) -only (CA) POM (UK) -only (US)
Routes Oral
Pharmacokinetic data
Bioavailability 4215%
Protein binding 272% (parent compound), 3012% (active metabolite, desvenlafaxine)
Metabolism Hepatic (~50% of the parent compound is metabolised on first pass through the liver)
Half-life 52 hours (parent compound for immediate release preparations), 156 hours (parent compound for extended release
preparations), 112 hours (active metabolite)
Excretion Renal (87%; 5% as unchanged drug; 29% as desvenlafaxine and 53% as other metabolites)
Identifiers
CAS number
93413-69-5
[3]
ATC code
N06AX16
[4]
PubChem
CID 5656
[5]
Venlafaxine
2
DrugBank
DB00285
[6]
ChemSpider
5454
[7]
UNII
GRZ5RCB1QG
[8]
ChEBI
CHEBI:9943
[9]
ChEMBL
CHEMBL637
[10]
Chemical data
Formula C
17
H
27
NO
2
Mol. mass 277.402 g/mol
(what is this?) (verify)
[11]
Venlafaxine (brand name: Effexor or Efexor) is an antidepressant of the serotonin-norepinephrine reuptake
inhibitor (SNRI) class. First introduced by Wyeth in 1993, now marketed by Pfizer, it is licensed for the treatment of
major depressive disorder (MDD), as a treatment for generalized anxiety disorder, and comorbid indications in
certain anxiety disorders with depression. In 2007, venlafaxine was the sixth most commonly prescribed
antidepressant on the U.S. retail market, with 17.2 million prescriptions.
[12]
Medical uses
Venlafaxine is used primarily for the treatment of depression, general anxiety disorder, social phobia, panic disorder
and vasomotor symptoms.
At low doses (<150mg/day), it acts only on serotonergic transmission. At moderate doses (>150mg/day), it acts on
serotonergic and noradrenergic systems, whereas at high doses (>300mg/day), it also affects dopaminergic
neurotransmission.
Many doctors are starting to prescribe venlafaxine "off label" for the treatment of diabetic neuropathy (in a similar
manner to duloxetine) and migraine prophylaxis (in some people, however, venlafaxine can exacerbate or cause
migraines). Studies have shown venlafaxine's effectiveness for these conditions. It has also been found to reduce the
severity of 'hot flashes' in menopausal women and men on hormonal therapy for the treatment of prostate cancer.
Substantial weight loss in patients with major depression, generalized anxiety disorder, and social phobia have been
noted, but the manufacturer does not recommend use as an anorectic either alone or in combination with
phentermine or other amphetamine-like drugs. Venlafaxine hydrochloride is in the phenethylamine class of modern
chemicals, which includes amphetamine, methylenedioxymethamphetamine (MDMA), and methamphetamine. This
chemical structure likely lends to its activating properties; however, some patients find venlafaxine highly sedating,
despite its more common stimulatory effects.
Venlafaxine is not recommended nor approved for the treatment of major depressive episodes in bipolar disorder as
it can induce mania or mixed episodes. Venlafaxine appears to be more likely than the SSRIs and bupropion to
induce mania and mixed episodes in bipolar patients.
Due to its action on both the serotoninergic and adrenergic systems, venlafaxine is also used as a treatment to reduce
episodes of cataplexy, a form of muscle weakness, in patients with the sleep disorder narcolepsy.
Venlafaxine was found in one study to be equal to clomipramine (anafranil) in the treatment of
Obsessive-compulsive disorder (OCD) with fewer side effects.
Some open-label and three double-blind studies have suggested the efficacy of venlafaxine in the treatment of
attention deficit-hyperactivity disorder (ADHD).
Venlafaxine
3
Due to its tendency to increase blood pressure and its modulative effects on the autonomic nervous system,
venlafaxine is often used to treat orthostatic intolerance and postural orthostatic tachycardia syndrome.
Depression
Multiple double blind studies show venlafaxine's effectiveness in treating depression. Venlafaxine has similar
efficacy to the tricyclic antidepressants amitriptyline (Elavil) and imipramine, and is better tolerated than
amitriptyline. Its efficacy is similar to or better than sertraline (Zoloft) and fluoxetine (Prozac), depending on the
criteria and rating scales used. Higher doses of venlafaxine are more effective, and more patients achieved remission
or were "very much improved". The efficacy was similar if the number of patients who achieved "response" or were
"improved" was considered. A meta-analysis comparing venlafaxine and combined groups of SSRI or tricyclic
antidepressants showed venlafaxine's superiority. Judged by the same criteria, venlafaxine was similar in efficacy to
the atypical antidepressant bupropion (Wellbutrin); however, the remission rate was significantly lower for
venlafaxine. In a double-blind study, patients who did not respond to an SSRI were switched to venlafaxine or
citalopram. Similar improvement was observed in both groups.
Adverse effects
Incidence of adverse effects
Very common (>10% incidence) adverse effects include:
Headache an often transient side effect that is common to most serotonin reuptake inhibitors and that most
often occurs at the beginning of therapy or after a dose escalation.
Nausea an adverse effect that is more common with venlafaxine than with the SSRIs. Usually transient and
less severe in those receiving the extended release formulations.
Insomnia
Asthenia (weakness)
Dizziness
Ejaculation disorder sexual side effects can be seen with virtually any antidepressant, especially those that
inhibit the reuptake of serotonin (including venlafaxine).
Somnolence
Dry mouth
Sweating
Common (110% incidence) adverse effects include:
Constipation
Nervousness
Abnormal vision
Anorgasmia
Hypertension
Impotence
Paresthesia
Tremor
Vasodilation
Vomiting
Weight loss
Chills
Palpitations
Confusion
Depersonalisation
Venlafaxine
4
Night sweats
Menstrual disorders associated with increased bleeding or increased irregular bleeding (e.g. menorrhagia,
metrorrhagia)
Urinary frequency increased
Abnormal dreams
Decreased libido
Increased muscle tonus
Yawning
Sweating
Abnormality of accommodation
Abnormal ejaculation/orgasm (males)
Urinary hesitancy
Serum cholesterol increased (especially when treatment is prolonged and it may be dose-dependent)
Uncommon (0.1-1% incidence) adverse effects include:
Face oedema
Intentional injury (self-harm)
Malaise
Moniliasis
Neck rigidity
Pelvic pain
Photosensitivity reaction
Suicide attempt
Withdrawal syndrome
Hypotension
Postural hypotension
Syncope
Tachycardia
Bruxism
Ecchymosis
Mucous membrane bleeding
Gastrointestinal bleeding
Abnormal liver function tests
Hyponatraemia
Weight gain
Apathy
Hallucinations
Myoclonus
Rash
Abnormal orgasm (females)
Urinary retention (the inability to pass urine)
Angioedema
Agitation
Impaired coordination & balance
Alopecia (hair loss)
Tinnitus (hearing bells)
Proteinuria (protein in urine)
Rare (0.010.1% incidence) adverse effects include:
Venlafaxine
5
Syndrome of inappropriate antidiuretic hormone secretion (SIADH)
Thrombocytopenia
Prolonged bleeding time
Seizures
Mania
Neuroleptic malignant syndrome (NMS)
Serotonin syndrome
Akathisia/psychomotor restlessness
Urinary incontinence
Very rare (<0.01% incidence) adverse effects include:
Anaphylaxis
QT prolongation
Ventricular fibrillation
Ventricular tachycardia (including torsade de pointes)
Pancreatitis
Blood dyscrasias (including agranulocytosis, aplastic anaemia, neutropenia and pancytopenia)
Elevated serum prolactin
Delirium
Extrapyramidal reactions (including dystonia and dyskinesia)
Tardive dyskinesia
Pulmonary eosinophilia
Erythema multiforme
Stevens-Johnson syndrome
Pruritus
Urticaria
Toxic epidermal necrolysis
Angle closure glaucoma
Suicide
The US Food and Drug Administration body (FDA) requires all antidepressants, including venlafaxine, to carry a
black box warning with a generic warning about a possible suicide risk.
A 2014 meta analysis of 21 clinical trials of venlafaxine for the treatment of depression in adults found that
compared to placebo, venlafaxine reduced the risk of suicidal thoughts and behavior.
A study conducted in Finland followed more than 15,000 patients for 3.4 years. Venlafaxine increased suicide risk
1.6-fold (statistically significant), as compared to no treatment. At the same time, fluoxetine (Prozac) halved the
suicide risk.
In another study, the data on more than 200,000 cases were obtained from the UK general practice research database.
At baseline, patients prescribed venlafaxine had a greater number of risk factors for suicide (such as prior suicide
attempts) than patients treated with other anti-depressants. The patients taking venlafaxine had significantly higher
risk of completed suicide than the ones on fluoxetine or citalopram (Celexa). After adjusting for known risk factors,
venlafaxine was associated with an increased risk of suicide relative to fluoxetine and dothiepin that was not
statistically significant. A statistically significant greater risk for attempted suicide remained after adjustment, but the
authors concluded that it could be due to residual confounding.
An analysis of clinical trials by the FDA statisticians showed the incidence of suicidal behaviour among the adults
on venlafaxine to be not significantly different from fluoxetine or placebo.
Venlafaxine
6
Venlafaxine is contraindicated in children, adolescents and young adults. According to the FDA analysis of clinical
trials venlafaxine caused a statistically significant 5-fold increase in suicidal ideation and behaviour in persons
younger than 25. In another analysis, venlafaxine was no better than placebo among children (711 years old), but
improved depression in adolescents (1217 years old). However, in both groups, hostility and suicidal behaviour
increased in comparison to those receiving a placebo. In a study involving antidepressants that had failed to produce
results in depressed teenagers, teens whose SSRI treatment had failed who were randomly switched to either another
SSRI or to venlafaxine showed an increased rate of suicide on venlafaxine. Among teenagers who were suicidal at
the beginning of the study, the rate of suicidal attempts and self-harm was significantly higher, by about 60%, after
the switch to venlafaxine than after the switch to an SSRI.
Dose dependency of adverse events
A comparison of adverse event rates in a fixed-dose study comparing venlafaxine 75, 225, and 375mg/day with
placebo revealed a dose dependency for some of the more common adverse events associated with venlafaxine use.
The rule for including events was to enumerate those that occurred at an incidence of 5% or more for at least one of
the venlafaxine groups and for which the incidence was at least twice the placebo incidence for at least one
venlafaxine group. Tests for potential dose relationships for these events (Cochran-Armitage test, with a criterion of
exact 2-sided p-value 0.05) suggested a dose-dependency for several adverse events in this list, including chills,
hypertension, anorexia (symptom), nausea, agitation, dizziness, somnolence, tremor, yawning, sweating, and
abnormal ejaculation.
Discontinuation syndrome
Main article: SSRI discontinuation syndrome
Patients stopping venlafaxine commonly experience SSRI discontinuation syndrome. The high risk of
discontinuation syndrome symptoms may reflect venlafaxine's short half-life.
Discontinuation is similar in nature, but not identical to those of SSRIs such as paroxetine (Paxil or Seroxat). Sudden
discontinuation of venlafaxine particularly seemed to cause discontinuation symptoms during the first 3 days in a
study of 18 patients. As reported in 2001 by Haddad in the journal Drug Safety, "another strategy to consider is
switching to fluoxetine, which may suppress the discontinuation symptoms, but which has little tendency to cause
such symptoms itself," and then discontinuing that.
Norepinephrine may also have a significant role in discontinuation symptoms. During withdrawal from venlafaxine,
the levels of both serotonin and norepinephrine decrease, rather than increase, and this would appear to rule out toxic
(too high) levels of these neurotransmitters as a likely cause of the withdrawal symptoms. The withdrawal symptoms
can be hypothesized to result from an overly rapid deprivation of neurotransmitter levels.
Serotonin syndrome
The development of a potentially life-threatening serotonin syndrome (also more recently classified as "serotonin
toxicity") may occur with venlafaxine treatment, particularly with concomitant use of serotonergic drugs, including
but not limited to SSRIs and SNRIs, many hallucinogens such as tryptamines and phenethylamines (e.g., LSD/LSA,
DMT, MDMA, mescaline), dextromethorphan(DXM)/dextrorphan (DXO), tramadol, tapentadol, pethidine
(meperidine) and triptans and with drugs that impair metabolism of serotonin (including MAOIs). Serotonin
syndrome symptoms may include mental status changes (e.g. agitation, hallucinations, coma), autonomic instability
(e.g. tachycardia, labile blood pressure, hyperthermia), neuromuscular aberrations (e.g. hyperreflexia,
incoordination) and/or gastrointestinal symptoms (e.g. nausea, vomiting, diarrhea). Venlafaxine-induced serotonin
syndrome has also been reported when venlafaxine has been taken in isolation in overdose. An abortive serotonin
syndrome state, in which some but not all of the symptoms of the full serotonin syndrome are present, has been
reported with venlafaxine at mid-range dosages (150mg per day) A case of a patient with serotonin syndrome
Venlafaxine
7
induced by low-dose venlafaxine (37.5mg per day) has also been reported.
Contraindications
Studies of venlafaxine in paediatric age groups have not established its efficacy. Venlafaxine is not recommended in
patients hypersensitive to it, nor should it be taken by anyone who is allergic to the inactive ingredients, which
include gelatin, cellulose, ethylcellulose, iron oxide, titanium dioxide and hypromellose. It should not be used in
conjunction with a monoamine oxidase inhibitor (MAOI), as it can cause potentially fatal serotonin syndrome.
Glaucoma
Venlafaxine can increase eye pressure, so those with glaucoma may require more frequent eye checks.
Pregnant women
There are few well-controlled studies of venlafaxine in pregnant women. A study released in May 2010 by the
Canadian Medical Association Journal suggests use of venlafaxine doubles the risk of miscarriage. Consequently,
venlafaxine should only be used during pregnancy if clearly needed. Prospective studies have not shown any
statistically significant congenital malformations. There have, however, been some reports of self-limiting effects on
newborn infants. As with other serotonin reuptake inhibitors (SRIs), these effects are generally short-lived, lasting
only 3 to 5 days, and rarely resulting in severe complications.
Drug interactions
Venlafaxine should be taken with caution when using St John's wort. Venlafaxine may lower the seizure threshold,
and coadministration with other drugs that lower the seizure threshold such as bupropion and tramadol should be
done with caution and at low doses.
Other
There have been false positive phencyclidine (PCP) results caused by larger doses of venlafaxine, with certain
on-site routine urine-based drug tests.
Overdose
Most patients overdosing with venlafaxine develop only mild symptoms. However, severe toxicity is reported, with
the most common symptoms being CNS depression, serotonin toxicity, seizure, or cardiac conduction abnormalities.
Venlafaxine seems to be more dangerous in overdose than the SSRIs, except perhaps citalopram which is more
dangerous than the other SSRIs in overdose. Despite this it appears less dangerous than bupropion, the tricyclic
antidepressants and the irreversible monoamine oxidase inhibitors. Deaths have been reported following very large
doses. Plasma venlafaxine concentrations in overdose survivors have ranged from 6 to 24mg/l, while postmortem
blood levels in fatalities are often in the 1090mg/l range.
On May 31, 2006, the Medicines and Healthcare Products Regulatory Agency (MHRA) UK concluded its review of
the latest safety evidence relating to venlafaxine, and particularly looked at the risks associated with overdose. The
advice was: the need for specialist supervision in those severely depressed or hospitalized patients who need doses
300mg or more; cardiac contraindications are more targeted towards high risk groups; patients with uncontrolled
hypertension should not take venlafaxine, and blood pressure monitoring is recommended for all patients; and
updated advice on possible drug interactions.
On 17 October 2006, Wyeth and the FDA notified healthcare professionals of revisions to the Overdosage/Human
Experience section of the prescribing information for Effexor (venlafaxine) indicated for treatment of major
depressive disorder. In post-marketing experience, there have been reports of overdose with venlafaxine, occurring
predominantly in combination with alcohol and/or other drugs. Published retrospective studies report that
Venlafaxine
8
venlafaxine overdosage may be associated with an increased risk of fatal outcome compared to that observed with
SSRI antidepressant products, but lower than that for tricyclic antidepressants. Healthcare professionals are advised
to prescribe Effexor and Effexor XR in the smallest quantity of capsules consistent with good patient management to
reduce the risk of overdose.
A report in the British Medical Journal in 2002 by Nicholas Buckley and colleagues at the Department of Clinical
Pharmacology and Toxicology, Canberra Hospital, Australia, studying fatal toxicity index (deaths per million
prescriptions), found that venlafaxine's fatal toxicity is higher than that of other serotoninergic antidepressants, but it
is similar to that of some of the less toxic tricyclic antidepressants. Overall, they found serious toxicity could occur
following venlafaxine overdose with reports of deaths, arrythmias, and seizures. They did, however, state that this
type of data is open to criticism, pointing out that mortality data may be influenced by previous literature and that
"less toxic" drugs may be preferentially prescribed to patients at higher risk of poisoning and suicide, but they are
also less likely to be listed as the sole cause of death from overdose. It also assumed that drugs are taken in overdose
with similar frequency and in similar amounts. They suggested "clinicians need to consider whether factors in their
patients reduce or compensate for this risk before prescribing venlafaxine."
The 27 February 2007 Vancouver Sun reported that the BC Drug and Poison Information Centre had alerted doctors
that the drug poses a significant risk of death from overdose, saying that venlafaxine "appears more toxic than it was
originally hoped". A doctor from the Department of Pharmacy Services College of Pharmacy, at the Medical
University of South Carolina, reported on the death of a 39-year-old patient with a 30g overdose. To put this into
perspective, a patient would have to take over 66 of the infrequently prescribed 450mg high dosage pills, or 400 of
the commonly prescribed 75mg pills.
Management of overdose
There is no specific antidote for venlafaxine, and management is generally supportive, providing treatment for the
immediate symptoms. Administration of activated charcoal can prevent absorption of the drug. Monitoring of
cardiac rhythm and vital signs is indicated. Seizures are managed with benzodiazepines or other anticonvulsants.
Forced diuresis, hemodialysis, exchange transfusion, or hemoperfusion are unlikely to be of benefit in hastening the
removal of venlafaxine, due to the drug's high volume of distribution.
Mechanism of action
Venlafaxine is usually categorized as a serotonin-norepinephrine reuptake inhibitor (SNRI), but it has been referred
to as a serotonin-norepinephrine-dopamine reuptake inhibitor (SNDRI). It works by blocking the transporter
"reuptake" proteins for key neurotransmitters affecting mood, thereby leaving more active neurotransmitters in the
synapse. The neurotransmitters affected are serotonin and norepinephrine. Additionally, in high doses it weakly
inhibits the reuptake of dopamine, with recent evidence showing that the norepinephrine transporter also transports
some dopamine as well, since dopamine is inactivated by norepinephrine reuptake in the frontal cortex. The frontal
cortex largely lacks dopamine transporters; therefore, venlafaxine can increase dopamine neurotransmission in this
part of the brain. Venlafaxine interacts with opioid receptors (mu-, kappa1- kappa3- and delta-opioid receptor
subtypes) as well as the alpha2-adrenergic receptor, and was shown to increase pain threshold in mice. When mice
were tested with a hotplate analgesia meter (to measure pain), both venlafaxine and mirtazapine induced a
dose-dependent, naloxone-reversible antinociceptive effect following intraperitoneal injection. These findings
suggest venlafaxine's seemingly superior efficacy in severe depression as narcotics become increasingly used as a
measure of last resort for refractory cases.
Venlafaxine
9
Pharmacokinetics
Venlafaxine is well absorbed, with at least 92% of an oral dose being absorbed into systemic circulation. It is
extensively metabolized in the liver via the CYP2D6 isoenzyme to desvenlafaxine (O-desmethylvenlafaxine), which
is just as potent a SNRI as the parent compound, meaning that the differences in metabolism between extensive and
poor metabolisers are not clinically important in terms of efficacy. Side effects, however, are reported to be more
severe in CYP2D6 poor metabolisers. Steady-state concentrations of venlafaxine and its metabolite are attained in
the blood within 3 days. Therapeutic effects are usually achieved within 3 to 4 weeks. No accumulation of
venlafaxine has been observed during chronic administration in healthy subjects. The primary route of excretion of
venlafaxine and its metabolites is via the kidneys. The half-life of venlafaxine is relatively short, so patients are
directed to adhere to a strict medication routine, avoiding missing a dose. Even a single missed dose can result in
withdrawal symptoms.
Venlafaxine is a substrate of P-glycoprotein (P-gp), which pumps it out of the brain. The gene encoding P-gp,
ABCB1, has the SNP rs2032583, with alleles C and T. The majority of people (about 70% of Europeans and 90% of
East Asians) have the TT variant. A 2007 study found that carriers of at least one C allele (variant CC or CT) are
7.72 times more likely than non-carriers to achieve remission after 4 weeks of treatment with amitriptyline,
citalopram, paroxetine or venlafaxine (all P-gp substrates). The study included patients with mood disorders other
than major depression, such as bipolar II; the ratio is 9.4 if these other disorders are excluded. At the 6-week mark,
75% of C-carriers had remitted, compared to only 38% of non-carriers.
Chemistry
The chemical structure of venlafaxine is designated (R/S)-1-[2-(dimethylamino)-1-(4 methoxyphenyl)ethyl]
cyclohexanol hydrochloride or ()-1-[a [a- (dimethylamino)methyl] p-methoxybenzyl] cyclohexanol hydrochloride,
and it has the empirical formula of C
17
H
27
NO
2
. It is a white to off-white crystalline solid. Venlafaxine is structurally
and pharmacologically related to the atypical opioid analgesic tramadol, and more distantly to the newly released
opioid tapentadol, but not to any of the conventional antidepressant drugs, including tricyclic antidepressants, SSRIs,
MAOIs, or RIMAs.
Available forms
Effexor XR 75 mg and 150 mg capsules
Extended release
Venlafaxine extended release is chemically the same as normal venlafaxine.
The extended release (controlled release) version distributes the release of the
drug into the gastrointestinal tract over a longer period than normal
venlafaxine. This results in a lower peak plasma concentration. Studies have
shown that the extended release formula has a lower incidence of patients
suffering from nausea as a side effect, resulting in a lower number of patients
stopping their treatment due to nausea. In Australia, New Zealand, Turkey
and Switzerland, Wyeth sells their venlafaxine XR tablets under the name
"Efexor-XR" (note the spelling with one 'f', rather than "Effexor-XR"). In
Brazil, Medley sells a venlafaxine XR capsule under the brand name Alenthus
XR. In September 2008, Osmotica Pharmaceuticals began marketing
venlafaxine extended release tablets in the United States to compete with Wyeth's capsule-form, Effexor-XR. Sales
of branded Efexor XR have
Venlafaxine
10
Generic 75mg (top) and 150mg (bottom)
venlafaxine capsules by Krka
remained strong, at US$2.7bn. Per settlement agreements, Teva and Impax
began offering generic Effexor XR in the US (with royalties paid to Wyeth);
Teva began on July 1, 2010, and Impax on July 1, 2011.
Generic
Generic venlafaxine is available in the United States as of August 2006 and in
Canada as of December 2006 due to patent expiry. Generic forms of the
extended-release version have been available in Canada as of January 2007
and currently include Co Venlafaxine XR (Cobalt Pharmaceuticals Inc.),
Gen-Venlafaxine XR (Genpharm), Riva-Venlafaxine XR (Laboratoire Riva
Inc.), Novo Venlafaxine XR (Novopharm Limited), PMS-Venlafaxine XR
(Pharmascience Inc.), Ratio-Venlafaxine XR (ratiopharm), Viepax (in Israel) and Sandoz Venlafaxine XR (Sandoz
Canada Inc.). Generic versions of both drug forms are available now in India and Australia. Generic products on the
South African market include Venlor SR Capsules (Cipla Medpro) and Illovex SR Tablets (Pharmadynamics, both
are available in 150mg and 75mg strengths. Generic versions are also available in the UK such as Vaxalin
manufactured by RatioPharm GmbH. On May 7, 2010 the Canadian pharmaceutical company IntelliPharmaCeutics
Inc. announced that the FDA had accepted its filing for a generic version of Venlafaxine XR utilizing its own
proprietary technologies.
References
[1] http:/ / www. drugs. com/ monograph/ venlafaxine. html
[2] http:/ / dailymed.nlm. nih. gov/ dailymed/ drugInfo.cfm?id=b23637e5-d37f-41b5-ba76-fc053e903bc2
[3] http:/ / www. nlm. nih.gov/ cgi/ mesh/ 2009/ MB_cgi?term=93413-69-5& rn=1
[4] http:/ / www. whocc.no/ atc_ddd_index/ ?code=N06AX16
[5] http:/ / pubchem. ncbi. nlm.nih. gov/ summary/ summary. cgi?cid=5656
[6] http:/ / www. drugbank. ca/ drugs/ DB00285
[7] http:/ / www. chemspider.com/ Chemical-Structure.5454. html
[8] http:/ / fdasis.nlm. nih. gov/ srs/ srsdirect. jsp?regno=GRZ5RCB1QG
[9] https:/ / www. ebi. ac. uk/ chebi/ searchId.do?chebiId=CHEBI:9943
[10] https:/ / www.ebi.ac. uk/ chembldb/ index.php/ compound/ inspect/ CHEMBL637
[11] http:/ / en. wikipedia. org/ w/ index. php?title=Special:ComparePages& rev1=459442662& page2=Venlafaxine
[12] [12] The number of prescriptions was calculated as the total of prescriptions for the corresponding generic and brand-name drugs using data from
the charts for generic and brand-name drugs.
External links
Drug information
U.S. Food and Drug Administration information on Effexor (http:/ / www. fda. gov/ Drugs/ DrugSafety/
PostmarketDrugSafetyInformationforPatientsandProviders/ ucm106481. htm)
Efexor patient information leaflet (UK) (http:/ / www. medicines. org. uk/ EMC/ medicine/ 8609/ XPIL/ Efexor+
XL/ )
Effexor XR prescribing information for healthcare professionals (pdf) (USA only) (http:/ / web. archive. org/
web/ 20060917025217/ http:/ / www. wyeth. com/ content/ ShowLabeling. asp?id=100) Archived from the
original (http:/ / www. wyeth. com/ content/ ShowLabeling. asp?id=100) on 17 September 2006.
Detailed Patient/Parent Information on Effexor (http:/ / www. rxlist. com/ cgi/ generic/ venlafax_pi. htm)
List of international brand names for Venlafaxine (http:/ / www. merck. com/ mmpe/ lexicomp/ venlafaxine.
html#N18219E)
Venlafaxine
11
U.S. National Library of Medicine: Drug Information Portal -Venlafaxine (http:/ / druginfo. nlm. nih. gov/
drugportal/ dpdirect. jsp?name=Venlafaxine)
Diagnostic tools
The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity (http:/
/ www. qjmed. oxfordjournals. org/ cgi/ content/ full/ 96/ 9/ 635)
Patient experiences
Stutz, Bruce "Self-Nonmedication" New York Times Magazine May 6, 2007 (http:/ / www. nytimes. com/ 2007/
05/ 06/ magazine/ 06antidepressant-t. html?_r=1& ei=5087 & em=& en=cdeb03773a3deee0& ex=1178596800&
pagewanted=all& oref=slogin)
Effexor Side Effects (http:/ / web. archive. org/ web/ 20130421232047/ http:/ / sideeffectsofeffexor. org)
Article Sources and Contributors
12
Article Sources and Contributors
Venlafaxine Source: http://en.wikipedia.org/w/index.php?oldid=612322981 Contributors: ABCD, AManWithNoPlan, Aaron Brenneman, Abdoytw, Acctorp, Acdx, Adamantios, Adeez, Agjchs,
Ail Subway, Alan Liefting, Alexrexpvt, Alfie66, Almazi, American2, AmiLynch, Amnesiac1977, Angel caboodle, Anticenaguy1, Aodidi, Aon, Arc101, Arcadian, AsceticRose, Assasinlv,
Athanatizein, Atxd00d, Audioiv, Avb, BD2412, Badgerbear, BalthCat, Barefootguy, Barrylb, Bdelisle, Bdve, Beetstra, Before My Ken, Belovedfreak, Benjah-bmm27, Benjaminevans82,
Between My Ken, Bignoter, Bkonrad, Boghog, Bookandcoffee, Bradley Parsons, Bwilliams, C.Fred, C6541, CKelly, Carlk4574, Carlo Banez, Casforty, Cashew, Casliber, Causa sui, Ceyockey,
Champlax, Charlene Copley, Chem-awb, Chemgirl131, Chowbok, Chris1900, ChrisGualtieri, Cimistus, Cjewell, Cleduc, Colin, CraigRNielsen, Crh8075, Curb Chain, Cybercobra, DMacks,
DanBealeCocks, Dandv, David Hedlund, Davidruben, Dawn Bard, Dead Midgets, Defenestration, Deli nk, Delta G, Demeter, DemonicPartyHat, DennyColt, Derek R Bullamore,
Devonspencer91, Dewritech, Dgw, DickK, Discospinster, DoctorEric, DougEngland, Douglasjoseph, Dr Zak, Draconis Neurocam, Dragadoo, DragonflySixtyseven, DreamsAreMadeOf,
Drpickem, Druid816, E dog96, Edgar181, Edmapa, Edonb, Eequor, Eet2006, EffexorFX, Elvey, Entoman, Eperotao, EuroCarGT, EvergreenFir, Exxolon, Fabiform, Fadix, Fallahi, Ferrierd,
Finetooth, Fluffernutter, Formerly 98, Fredrik, FreplySpang, Fribbler, Frze, Fsk, Fuse809, GVP444, Gaius Cornelius, Genrethan, Ghettodev, Giftlite, Gigemag76, Gilliam, Ginkgo100,
Ginsengbomb, Glover, Gman620, Gmmm2005, Googie man, GraemeL, Grugnir, Harbinary, HazyM, Headbomb, Hecatonchireslm, Hopkapi, InfiniteWisdom, IrisKawling, Ironjb, Ithacagorges,
Ixfd64, J, JLaTondre, JPX7, Jab843, Jamesofur, Jb4671, Jenblower, Jesster79, Jfdwolff, Jmax-, Jmh649, John Cho, JohnSRoberts99, Johnny 0, Joshjaeger, Jrun, Jscedaz, Jtauxier, Julcal, Justin W
Smith, J, Kazlow101, Keirano, Khazar2, Kilom691, Ksu6500, Kwilson, Landty, Lawn, Layraud, Licon, Ligulem, Lilac Soul, LittleHow, Looie496, Luna Santin, Lupin, Lyrl, MadSurgeon,
Magioladitis, Maima, Mana Excalibur, Mary Heath, Mat8989, Materialscientist, Matthew Proctor, Maurog, MeekMark, Meodipt, Meteor sandwich yum, Miami33139, Michael.j.lacey,
MikeAllen, Milonica, Mircetic, MistyHora, Moop stick, Mr Bungle, MrADHD, MrBill3, MrPlaid81, Muugokszhiion, NBINVDR1, Nat Radzi, NateJames, Navicular, Nbauman, Nevit, Nihiltres,
Noisy Crew, Nono64, NotAnIP83:149:66:11, Nuklear, Nutriveg, Nwbeeson, O.mangold, Octavio L, Ohnoitsjamie, Olathe, Omega85, Omegatron, One Salient Oversight, Oobopshark, Orbst,
Otisbd, Oxfordwang, Pacula, Padswaggle, Parhamr, Paul gene, Pearle, PhnomPencil, Pne, Prisonnet, ProBonoPublicoA90, ProperlyRaised, Psykoosi, Ptcamn, Purple parrot, Qlez, Quercus solaris,
Quiddity, Qwfp, R'n'B, Ravellwolf, Rbaselt, Reedy, Refael Ackermann, Remark knights, Rhodekyll, Rholton, Rich Farmbrough, Rickcrane, Rjwilmsi, Rkmlai, Rmky87, Roger Roger,
Rsimon7632, SDC, Sarahmagicbadger, Schneelocke, Seamorgh, Secretlondon, Selket, Semilanceata, Shisha-Tom, Shoy, SixSix, Slowking Man, Smb1138, Smcauley, Soakologist, Spyros
Pantenas, Ssuway, Staciabb, Stepa, Stillwaterising, Stratman07, Stu963, Sue Rangell, Sunborn, Supergloom, Supersox, Supertess82, Svgalbertian, SynapticPaul, Szimonsays, TVC 15, Tatterfly,
Tdonner, Tentinator, Terschinbrae, That Guy, From That Show!, The Sceptical Chymist, Thecheesykid, Thixotropic42, Timeshift9, Tinkog, Trivialist, Trovatore, Twas Now, Tybalt01, TyrS,
Ukexpat, Untrue Believer, Uthbrian, Utsuprainfra, VICVONWIKVIT, Vaccinationist, Vanished user kasjqwii3km4tkid, Vardyr, Wahwahpedal, Wavelength, Wayward, Webclient101, Wikiborg,
Williadb, Wimt, Wisconsinator, Wku2m5rr, Woohookitty, Xenobiologista, Z0manifest, Zeppelin4life, ZimZalaBim, Zuiram, , 593 anonymous edits
Image Sources, Licenses and Contributors
File:Venlafaxine structure.svg Source: http://en.wikipedia.org/w/index.php?title=File:Venlafaxine_structure.svg License: Public Domain Contributors: User:Vaccinationist
File:Venlafaxine3Dan2.gif Source: http://en.wikipedia.org/w/index.php?title=File:Venlafaxine3Dan2.gif License: Creative Commons Attribution-Sharealike 3.0 Contributors: Fuse809
File:Yes check.svg Source: http://en.wikipedia.org/w/index.php?title=File:Yes_check.svg License: Public Domain Contributors: Anomie
Image:EffexorXR 75and150mg.png Source: http://en.wikipedia.org/w/index.php?title=File:EffexorXR_75and150mg.png License: Public Domain Contributors: Parhamr
Image:Venlafaxin Krka.jpg Source: http://en.wikipedia.org/w/index.php?title=File:Venlafaxin_Krka.jpg License: Public Domain Contributors: Aon
License
Creative Commons Attribution-Share Alike 3.0
//creativecommons.org/licenses/by-sa/3.0/
You might also like
- Eti Nurwening Sholikhah: Department of Pharmacology & Therapy Faculty of Medicine Universitas Gadjah MadaDocument43 pagesEti Nurwening Sholikhah: Department of Pharmacology & Therapy Faculty of Medicine Universitas Gadjah MadaadystiNo ratings yet
- Psycho-Pharmacotherapy: Major Tranquilizers, D2 - Receptor Blockers and Anti - Schizophrenic DrugsDocument29 pagesPsycho-Pharmacotherapy: Major Tranquilizers, D2 - Receptor Blockers and Anti - Schizophrenic DrugsPoonam RanaNo ratings yet
- Principles of PharmacotherapyDocument40 pagesPrinciples of Pharmacotherapyjunitria13No ratings yet
- Pharmacotherapy for schizophrenia: Acute and maintenance treatmentDocument17 pagesPharmacotherapy for schizophrenia: Acute and maintenance treatmentNadya SaptarinaNo ratings yet
- Drug StudyDocument10 pagesDrug StudyRubie Ann TillorNo ratings yet
- All Other ClassificationsDocument6 pagesAll Other ClassificationsCorey100% (1)
- Atypical Antidepressants - Pharmacology, Administration, and Side Effects - UpToDateDocument16 pagesAtypical Antidepressants - Pharmacology, Administration, and Side Effects - UpToDateMelissandreNo ratings yet
- Carmencita R. Pacis, RN Man PHDDocument35 pagesCarmencita R. Pacis, RN Man PHDkristelaaa guevarraNo ratings yet
- Vitamin B-complex and C supplement capsulesDocument2 pagesVitamin B-complex and C supplement capsulessoumalya4810% (1)
- Tramadol HydrochlorideDocument157 pagesTramadol HydrochlorideShahid LatifNo ratings yet
- Chronic Addiction To Dextromethorphan Cough Syrup: A Case ReportDocument4 pagesChronic Addiction To Dextromethorphan Cough Syrup: A Case ReportEgy Saputra JayaNo ratings yet
- CNS StimulantDocument26 pagesCNS StimulantIslam Abdo50% (2)
- Drug Utilization Review (DUR)Document8 pagesDrug Utilization Review (DUR)Rinta MoonNo ratings yet
- Toxicology in The Drug Discovery and Development Process: UNIT 10.3Document35 pagesToxicology in The Drug Discovery and Development Process: UNIT 10.3Nilabh RanjanNo ratings yet
- Drugs Affecting The Central Nervous SystemDocument33 pagesDrugs Affecting The Central Nervous SystemEeric AlexanderNo ratings yet
- Anxiety Disorders Treatment OptionsDocument5 pagesAnxiety Disorders Treatment OptionsJohn HolmesNo ratings yet
- Practical Manua PDFDocument25 pagesPractical Manua PDFIbrahim ShakirNo ratings yet
- Mechanism of Action of QuetiapineDocument4 pagesMechanism of Action of QuetiapineThoha AlbaarNo ratings yet
- Medicinal Chemistry-Ii: 1.anti-Infective Agents: FDocument14 pagesMedicinal Chemistry-Ii: 1.anti-Infective Agents: FAnonymous ionOPaqlkNo ratings yet
- Introduction To Acute and Ambulatory Care Pharmacy PracticeDocument100 pagesIntroduction To Acute and Ambulatory Care Pharmacy PracticeDanielle De GuzmanNo ratings yet
- Top 200 Drugs Class/Use GuideDocument4 pagesTop 200 Drugs Class/Use GuideEsther AhnNo ratings yet
- Anxiolytics and Hypnotics by Sue HendersonDocument8 pagesAnxiolytics and Hypnotics by Sue HendersonJoyabrata SarkarNo ratings yet
- Prozac (Fluoxetine) 40mgDocument1 pageProzac (Fluoxetine) 40mgENo ratings yet
- Katzung Chapter 24 Antiseizure DrugsDocument52 pagesKatzung Chapter 24 Antiseizure DrugsMeetAndreaNo ratings yet
- Compounding in Community SettingDocument19 pagesCompounding in Community Settingkhangsiean89100% (1)
- Revellionz'19 - Second Year Question BankDocument114 pagesRevellionz'19 - Second Year Question BankRamNo ratings yet
- Adrenergic Blocking, Cholinergic, Sedatives and Hypnotic WORDDocument5 pagesAdrenergic Blocking, Cholinergic, Sedatives and Hypnotic WORDKeon RicoNo ratings yet
- Tramadol OMSDocument39 pagesTramadol OMSFranco VairolettiNo ratings yet
- Treatment of Common SymptomsDocument12 pagesTreatment of Common Symptomsxjunhao100% (3)
- Guidelines+ +Opioids+ChapterDocument16 pagesGuidelines+ +Opioids+ChapterRajinder Kumar BassanNo ratings yet
- Patient Medication Profile and CounselingDocument56 pagesPatient Medication Profile and CounselingMeimei QueNo ratings yet
- Jurnal BioadhesifDocument10 pagesJurnal BioadhesifIzhal StewartNo ratings yet
- Part Agents Act NG On The Central Ner Ous System: Liu JuntianDocument89 pagesPart Agents Act NG On The Central Ner Ous System: Liu Juntianapi-19916399No ratings yet
- Ciplamed - Montair LC & Montair LC Kid DT - Syrup - 2018-03-20Document11 pagesCiplamed - Montair LC & Montair LC Kid DT - Syrup - 2018-03-20anon_458167643100% (1)
- Cns Stimulants: (MOA and Uses)Document39 pagesCns Stimulants: (MOA and Uses)Mirza Shaharyar BaigNo ratings yet
- Adult Parenteral Guidelines 2020Document57 pagesAdult Parenteral Guidelines 2020Sara Aly YoussefNo ratings yet
- Treating urinary infection with cefuroximeDocument15 pagesTreating urinary infection with cefuroximeKate Penelope DalidNo ratings yet
- Psychopharmacology NewestDocument43 pagesPsychopharmacology NewestRegina PunNo ratings yet
- Dextroamphetamine SulfateDocument3 pagesDextroamphetamine Sulfateapi-3797941100% (1)
- Introduction To Pharmacoepidemiology 2015 PDFDocument20 pagesIntroduction To Pharmacoepidemiology 2015 PDFNovria Rizki HarahapNo ratings yet
- Antipsychotic DrugsDocument47 pagesAntipsychotic DrugsIkram UddinNo ratings yet
- ZoloftDocument26 pagesZoloftgofastjayNo ratings yet
- Bronchodilator & Other Drugs Used in AsthmaDocument15 pagesBronchodilator & Other Drugs Used in AsthmaGenta JagadNo ratings yet
- Medication HistoryDocument19 pagesMedication HistoryAnisha PandeyNo ratings yet
- Antipsychotic Medication: Generic Name Trade Name Indications Contraindications Drug Interaction Side Effects Nursing ImplicationDocument6 pagesAntipsychotic Medication: Generic Name Trade Name Indications Contraindications Drug Interaction Side Effects Nursing ImplicationJaylord VerazonNo ratings yet
- Chapter 6Document27 pagesChapter 6yeshi janexo100% (1)
- Pharmacist's Letter: Prescriber's LetterDocument6 pagesPharmacist's Letter: Prescriber's LetterJaved AkhtarNo ratings yet
- Treatment Modalities For Mood DisordersDocument55 pagesTreatment Modalities For Mood DisordersGlory MimiNo ratings yet
- Pharmacists Role Clinical Pharmacokinetic MonitoringDocument2 pagesPharmacists Role Clinical Pharmacokinetic MonitoringauliaNo ratings yet
- Challenges To Implementation of The Pharmaceutical Care Practice in Davao City.Document11 pagesChallenges To Implementation of The Pharmaceutical Care Practice in Davao City.JessieLynMolinaNo ratings yet
- Adverse Drug Reactions NotesDocument4 pagesAdverse Drug Reactions NoteskarthikeyanpgtNo ratings yet
- ParacetamolDocument78 pagesParacetamolMichalNo ratings yet
- S-GA Use in Pregnancy Linked to Increased RisksDocument9 pagesS-GA Use in Pregnancy Linked to Increased RisksDian Oktaria SafitriNo ratings yet
- Drug Dosage Forms II (PHR 312) Course OverviewDocument12 pagesDrug Dosage Forms II (PHR 312) Course OverviewprinceamitNo ratings yet
- Daptomycin (Cubicin)Document1 pageDaptomycin (Cubicin)Adrianne BazoNo ratings yet
- Psychiatric Agents: By: Paula Rose Mae Cuario Evita Lalaine Del Mundo Dennis Ragudo Sheena ZarsueloDocument80 pagesPsychiatric Agents: By: Paula Rose Mae Cuario Evita Lalaine Del Mundo Dennis Ragudo Sheena ZarsueloDennis RagudoNo ratings yet
- Nanoparticles in Cancer Therapy and DiagnosisDocument55 pagesNanoparticles in Cancer Therapy and Diagnosismimshin0% (1)
- Prescription Analysis1Document21 pagesPrescription Analysis1Rizzalaine CaringalNo ratings yet
- Case Study SaladDocument7 pagesCase Study SaladrimNo ratings yet
- AbacavirDocument5 pagesAbacavirMariusNeicuNo ratings yet
- Leonhard EulerDocument15 pagesLeonhard EulerMariusNeicuNo ratings yet
- Pharmaco KineticsDocument14 pagesPharmaco KineticsMariusNeicuNo ratings yet
- LorazepamDocument13 pagesLorazepamMariusNeicuNo ratings yet
- OlanzapineDocument12 pagesOlanzapineMariusNeicuNo ratings yet
- Lam IV UdineDocument5 pagesLam IV UdineMariusNeicuNo ratings yet
- BromazepamDocument6 pagesBromazepamMariusNeicuNo ratings yet
- AmoxicillinDocument8 pagesAmoxicillinMariusNeicuNo ratings yet
- Queti A PineDocument12 pagesQueti A PineMariusNeicuNo ratings yet
- AmpicillinDocument4 pagesAmpicillinMariusNeicuNo ratings yet
- Aspirin Paracetamol CaffeineDocument3 pagesAspirin Paracetamol CaffeineMariusNeicuNo ratings yet
- Levo Me PromazineDocument4 pagesLevo Me PromazineMariusNeicuNo ratings yet
- HaloperidolDocument12 pagesHaloperidolMariusNeicuNo ratings yet
- HESI Study Guide Psychiatric NursingDocument26 pagesHESI Study Guide Psychiatric NursingDean Winchester100% (4)
- Final Outline (Psychiatric Nursing)Document37 pagesFinal Outline (Psychiatric Nursing)Yucef Bahian-AbangNo ratings yet
- Mastery of Your AnxietyDocument182 pagesMastery of Your Anxietylaintrix046696100% (13)
- Case-Report ArticleDocument5 pagesCase-Report ArticleAnwar SamNo ratings yet
- Antidepressants and Sexual DysfunctionDocument15 pagesAntidepressants and Sexual DysfunctionGe NomNo ratings yet
- FmCases SummariesDocument94 pagesFmCases SummariesTauhid MahmudNo ratings yet
- Ssri Presentation - 674 - Final2Document52 pagesSsri Presentation - 674 - Final2api-289842236100% (1)
- Managing Behavioral Emergencies in Pregnant PatientsDocument14 pagesManaging Behavioral Emergencies in Pregnant PatientsAna María Arenas DávilaNo ratings yet
- Notes File - MergedDocument99 pagesNotes File - MergedMian. Shoaib.No ratings yet
- Psychiatric Nursing Pnle 2022Document25 pagesPsychiatric Nursing Pnle 2022Bariwan FaredaNo ratings yet
- Aripiprazole Lai Prescribing GuidelinesDocument8 pagesAripiprazole Lai Prescribing Guidelinestabic68932No ratings yet
- NCLEX Medications For Nurses 1 PDFDocument118 pagesNCLEX Medications For Nurses 1 PDF281175100% (4)
- الادوية فى مصر مرتبة بالاسم العلمى وبالتصنيفDocument112 pagesالادوية فى مصر مرتبة بالاسم العلمى وبالتصنيفmahgad83% (24)
- Dextroamphetamine: Brand Name: DexedrineDocument23 pagesDextroamphetamine: Brand Name: DexedrineSharry Fe OasayNo ratings yet
- Antiadrenergic-Hypotension Antimuscarinic (Anticholinergic) - Dry Mouth, BlurredDocument8 pagesAntiadrenergic-Hypotension Antimuscarinic (Anticholinergic) - Dry Mouth, Blurredwafaa alwafiNo ratings yet
- New Insights On Premature EjaculationDocument8 pagesNew Insights On Premature EjaculationTata VioNo ratings yet
- The Case of White Rabbit of Alice in The Wonderland 3Document6 pagesThe Case of White Rabbit of Alice in The Wonderland 3Paul AntolinoNo ratings yet
- Ann Blake SSRI DepressionDocument17 pagesAnn Blake SSRI Depressionthings3100% (1)
- Psychotropic MedicationDocument28 pagesPsychotropic Medicationrmconvidhya sri2015No ratings yet
- Postpartum DepressionDocument20 pagesPostpartum Depressiondrhusnafadzil83% (6)
- University of Southern Mindanao Personality DisordersDocument62 pagesUniversity of Southern Mindanao Personality DisorderssarocamkentNo ratings yet
- Drug-Induced Sleepiness and Insomnia: An Update: Sonolência e Insônia Causadas Por Drogas: Artigo de AtualizaçãoDocument8 pagesDrug-Induced Sleepiness and Insomnia: An Update: Sonolência e Insônia Causadas Por Drogas: Artigo de AtualizaçãoRene FernandesNo ratings yet
- Organic Chemistry ProjectDocument11 pagesOrganic Chemistry Projectapi-463768793No ratings yet
- Psych Drugs NursingDocument7 pagesPsych Drugs Nursinglisa100% (2)
- Individual Mental Health AssignmentDocument18 pagesIndividual Mental Health AssignmentRebekah Roberts100% (1)
- Social Anxiety DisorderDocument10 pagesSocial Anxiety DisorderLintang SuroyaNo ratings yet
- Depressed Diane-Case Study On DepressionDocument27 pagesDepressed Diane-Case Study On DepressionFarhath Jabien100% (1)
- Personal View: Mark Abie Horowitz, David TaylorDocument9 pagesPersonal View: Mark Abie Horowitz, David TaylorNoel Saúl Argüello SánchezNo ratings yet
- Mental health nursing techniques for psychiatric careDocument43 pagesMental health nursing techniques for psychiatric careHaru100% (1)
- DrugsDocument113 pagesDrugsCARE CATH LABNo ratings yet