Professional Documents
Culture Documents
Menkes Disease: Report of Two Cases
Uploaded by
rickyferdianOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Menkes Disease: Report of Two Cases
Uploaded by
rickyferdianCopyright:
Available Formats
Iran J Pediatr
Dec 2007; Vol 17 ( No 3), Pp:388-392
Case Report
Menkes Disease: Report of Two Cases
Mohammad Barzegar*1, MD; Afshin Fayyazie1, MD; Bobollah Gasemie2, MD;
Mohammad ali Mohajel Shoja3, MD
1. Pediatric Neurologist, Department of Pediatrics, Tabriz University of Medical Sciences, IR Iran
2. Pathologist, Department of Pathology, Tabriz University of Medical Sciences, IR Iran
3. General Physician, Tabriz University of Medical Sciences, IR Iran
Received: 14/05/07; Revised: 20/08/07; Accepted: 10/10/07
Abstract
Introduction: Menkes disease is a rare X-linked recessive disorder of copper metabolism. It is
characterized by progressive cerebral degeneration with psychomotor deterioration, hypothermia,
seizures and characteristic facial appearance with hair abnormalities.
Case Presentation: We report on two cases of classical Menkes disease with typical history,
(progressive psychomotor deterioration and seizures}, clinical manifestations (cherubic appearance,
with brittle, scattered and hypopigmented scalp hairs), and progression. Light microscopic
examination of the hair demonstrated the pili torti pattern. The low serum copper content and
ceruloplasmin confirmed the diagnosis.
Conclusion: Menkes disease is an under-diagnosed entity, being familiar with its manifestation and
maintaining high index of suspicion are necessary for early diagnosis.
Key Words: Menkes disease, Copper metabolism, Epilepsy, Pili torti, Cerebral degeneration
Introduction
Menkes disease (MD), also referred to as kinky
hair disease, trichopoliodystrophy, and steely hair
disease, is a rare X-linked recessive disorder of
copper metabolism.[1,2] It is characterized by
progressive cerebral degeneration with psychomotor deterioration, seizures, and connective
tissue alteration with hair abnormalities.[2,3] The
most striking finding is the appearance of the
scalp hair, being thin, coarse, brittle and
colorless. Examination under microscope reveals
a variety of abnormalities, most often pili torti
(twisted hair), monilethrix (varying diameter of
hair shafts) and trichorrhexis nodosa (fractures of
the hair shaft at regular intervals)[4]. The clinical
picture is caused by a defect in copper
transporting ATPase (ATP7A), resulting in
defects of key copper dependent enzymes,
including lysyl oxydase, cytochrome c oxidase,
dopamine -hydroxylase, tyrosinase, and super
* Correspondence author;
Address: Pediatric Department, Tabriz Children Hospital, Sheshgelan St, Tabriz, IR Iran
E-mail: mm_barzegar@yahoo.com
389
Iran J Pediatr. Vol 17 (No 4); Dec 2007
oxide dismutase. Depigmentation of hair and skin
pallor are due to tyrosinase deficiency,
hypothermia is due to cytochrome c oxidase
deficiency and lysyl oxidase deficiency causes
tortuous arteries in brain, progressive vascular
changes predispose to thrombosis and deficient
blood supply to the developing brain[2,3,5].
Neuroimaging discloses atrophy and bilateral
ischemic lesions in deep gray matter or in the
cortical areas; the consequence of vascular
infarctions.[7]
Management of patients with MD is
supportive, with an emphasis on anticonvulsant
treatment and a trial of copper histidine therapy.
Prognosis is poor with progressive neurological
deterioration and eventual death within the first 3
years of life.[3]
The clinical history and the appearance of the
infant should suggest the diagnosis. Microscopic
examination of the hair is very helpful even in a
mild case. Low levels of serum copper and
ceruloplasmin will usually confirm the
diagnosis.[5] If doubt still exists, the diagnosis can
be confirmed by demonstrating the intracellular
accumulation of copper and decreased efflux of
64
Cu from cultured fibroblasts.[6] Menkes disease
is a rare disorder; its frequency has been
estimated 1 in 114000-250000 live births.[8]
We report on two cases of classic MD
diagnosed in Tabriz Children's Hospital, a
university- affiliated tertiary hospital in the East
Azarbaijan province, the North West of Iran
between years 2002 and 2006.
control. Light microscopic examination of the
scalp hair showed pili torti. The diagnosis of MD
was confirmed on the basis of low serum copper
(15 g/dl; ref. range: 70-150 g/dl) and low
serum ceruloplasmin (58 mg/l; ref. range: 187322, mg/l). There were no abnormalities in other
standard blood analyses. The electroencephalography showed multifocal spikes and waves with
poorly organized sleep features. Brain CT scan
demonstrated cerebral atrophy and subdural
effusion. Copper-histidine was prescribed.
Despite anticonvulsant therapy with various
drugs (phenobarbital, clonazepam, nitrazepam,
vigabatrin) intractable seizures continued. At the
end of the first year of life, neurological
milestones such as head control, rollover
response and laughing had not been achieved.
Unfortunately, the patient died of a respiratory
infection at the age of 14 months.
Case(s) Presentation
Case 2: The five month-old male infant was
referred to our hospital with regression of
developmental milestones and seizures. He was
born at term to healthy consanguineous parents.
The pregnancy was uneventful. At birth the head
circumference and body weight were 35 cm and
3.2 kg, respectively. Family history was
remarkable for the death of two previous male
siblings at the age of 1 month and 18 months.
They had severe neurodevelopmental delay
without a definite diagnosis. The patient had a
history of 8 days hospital admission on third day
of life with poor feeding, hypothermia and
hyperbilirubinemia (total bilirubin was 16 mg/dl).
Case 1: A seven month-old male infant was
brought to our out-patient clinic due to gradualonset of hypotonia and seizures. The boy was
born at 34 weeks of gestational age to healthy,
non-consanguineous parents. He was the first
child of the parents. His early development was
age appropriate for 3 months, and then regressed.
At 5 months of age myoclonic jerks were noted.
His clinical examination at 7 months revealed
cherubic appearance with depressed nasal bridge,
and brittle, scattered and hypopigmented scalp
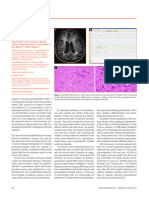
hairs (Fig 1). He had no eye contact and no head
Fig 1- Scattered and hypopigmented scalp hairs
in Menkes disease
390
Menkes Disease. M Barzegar, et al
His early development was age appropriate for 4
months, at 3 months of age he had good head
control and laughing. At 4 months of age tonic
and myoclonic seizures were noted. On clinical
examination at 5 months, the most striking
finding was the appearance of the scalp hair. It
was colorless, thin, brittle and kinky. Although
eye contact was noted, he had poor head control
and no rollover response. Brain CT scan showed
cerebral atrophy and subdural effusion. An
electroencephalography
revealed
frequent
multifocal
epileptiform
discharges
with
disorganized background. With high suspicion of
MD, serum copper and ceruloplasmin were
determined; with 3 g/dl (ref. 70-155) and 15
mg/l (ref. 187-320 mg/l) respectively both were
below normal levels. Light microscopic
examination of the hair showed pili torti (twisted
hair shafts) and trichorrhexis nodosa. There were
no metaphyseal changes of long bones on X-rays.
There were also no abnormalities in other
standard blood analyses. The diagnosis of MD
was made. Phenobarbital and nitrazepam were
partially effective. Poor weight gain, seizures and
neurologic deterioration were evident in a visit at
1 year of age. At 17 months of age he showed
severe global developmental delay and failure to
thrive. He has had two short hospital admissions
for chest infection and diarrhea.
Discussion
The clinical features and inheritance of MD were
first described in 1962.[9] Ten years later the
underlying biochemical defect in copper
metabolism was discovered.[10] The clinical
spectrum of MD encompasses several distinct
variants. The neonatal form is characterized by
multiple fractures and extensive vascular disease
with early death[11]. Infants with classic MD
typically appear healthy until 2 to 3 months of
age. Premature delivery is very frequent, as are
neonatal hypothermia and hyperbilirubinemia.
Hypothermia may also occur in older infants.
Neonatal symptoms may resolve, and the babies
may seem normal during next 2 or 3 months. At
3 months of age they start to demonstrate
developmental delay, hypotonia, intractable
seizures and failure to thrive. Cerebral
degeneration then dominates the clinical
picture.[1,3,5]
Children often have a cherubic appearance
with sparse, course, short, twisted, and lightly
pigmented hair. Individuals with the mild variant
are developmentally delayed with cerebellar
ataxia, dysarthria and pili torti, and no seizures.
The occipital horn syndrome is considered a MD
variant. The skeletal dysplasia, soft bruisable
skin, hyper-extensible joints, diarrhea, and
occipital exostosis characterize it.[12]
The typical history and clinical features in our
patients were suggestive of classical MD. The
cherubic facial appearance with a depressed nasal
bridge in both of them was similar to reported
cases in the past.[1-3,5,6,9,13]
Hair abormalities are the most striking signs
in this syndrome. Our patients showed brittle,
scattered and hypopigmented scalp hairs; under
microscope hair was shown to be twisted
longitudinally with narrowing at intervals alongwith many erosions on the hair shaft. The hair
was fragile and fractured easily, resulting in
apparent generalized alopecia. Several hair shaft
abnormalities have been documented, with pili
torti being the most common, also trichorrehexis
nodosa, trichoclasis, and trichoptilosis have been
reported.[4,14] The scalp hair may appear normal
at birth, but at approximately three months of age
the hair on the scalp and eyebrows becomes
kinky, coarse, and lightens in color.[1,3,5]
Nonskin manifestations in our patients were
delayed developmental milestones and intractable
seizures similar to other reports.[1,3,5,6,9] Epilepsy
is a frequent and early feature in MD, it was
reported in our cases. Myoclonous is the usual
seizure type; other types of seizures, including
multifocal seizure and tonic spasms are also
reported. Seizures are usually resistant to
antiepileptic drugs. The pathophysiologic
mechanisms of epilepsy in MD remain unknown,
but they are likely related to copper deficiency. It
results in an impairment of lysyl oxidase,
considered as the primary cause of the abnormal
intracranial vessel structures.[15]
Our patients had low serum copper and
ceruloplasmin levels which correlated with the
clinical findings, these levels are usually low but
interpretation may be difficult in the first few
391
Iran J Pediatr. Vol 17 (No 4); Dec 2007
months of life[5]. In the past, the final diagnosis
was made by cultured skin fibroblasts and
lymphoblasts,
which
showed
impaired
metabolism of copper.[6] Today this method is
being replaced by molecular genetic analysis,
available in certain laboratories, to confirm the
diagnosis for carrier testing or for prenatal
diagnosis.[16]
Our diagnosis was made on the basis of
clinical and laboratory findings. Our cases
fulfilled the following clinical and biologic
diagnostic features of the classical MD:
Progressive neurologic disease with marked
hypotonia and neurologic regression, feeding
difficulties with failure to thrive, cherubic
appearance with a depressed nasal bridge, brittle,
scattered and hypopigmented scalp hairs,
pathognomonic hair abnormalities consistent
with pili torti, and low plasma copper and serum
ceruloplasmin levels.
Copper histidine is the most effective
treatment for MD, and if admiminstered soon
after birth, neurological development can be
maintained[17]. By contrast, there are few
neurological
benefits
when
Cu-histidine
treatment is initiated after 2 months of age.[18]
Therefore it would be important to have it easily
available in drug market. The early diagnosis is
also mainly required for the genetic counseling.
The pattern of inheritance is X-linked with a
recurrence risk of 50% for the affected sons and
50% of daughters will be carriers. Unfortunately,
in our second case genetic analysis was not
available. Prenatal diagnosis of MD can be
performed by gene analysis and/or measurement
of the copper concentration in culture of amniotic
liquid cells and chorionic villi cells.[16]
Conclusion
We believe that MD is an under-diagnosed entity
in the developing countries, so being familiar
with its manifestation and maintaining high index
of suspicion are necessary for early diagnosis.
The diagnosis can be made with great confidence
by the typical clinical history and the appearance
of the infant once one or two cases have been
seen.
References
1. Menkes JH. Kinky hair disease, Pediatrics
1972;50(2):181-3.
2. Danks DM, Campbel PE, Walker-Smith J
et al. Menkes kinky-hair syndrome.
Lancet. 1972;1(7808):1100-2.
3. Menkes JH. Kinky hair disease: twenty
five years later. Brain Dev. 1998;10(2):
77-9.
4. Smith VV, Anderson G, Malone M, et al.
Light microscopic examination of scalp
hair samples as an aid in the diagnosis of
pediatric disorders: retrospective review of
more than 300 cases from a single centre. J
Clin Pathol. 2005;58(12):1294-8.
5. Menkes JH, Wilcox WR. Inherited
metabolic disease of the nervous system.
In: Menkes JH, Sarnat HB, Maria BL
(eds).
Child
Neurology.
7th
ed.
Philadeiphia: Lippimcott Williams &
Wilkins. 2006; Pp:115-7.
6. Tumer Z, Horn N. Menkes disease: recent
advances and new aspects. J Med Genet.
1997;34(4):265-74.
7. Ichihashi K, Yano S, Kobayashi S, et al.
Serial imaging of Menkes disease.
Neuroradiology. 1990;32(1):56-9.
8. Tonnesen T, Kleijer WJ, Horn N.
Incidence of Menkes disease. Hum Genet.
1991;86(4):408-10.
9. Menkes JH, Alter M, Steigleder GK, et al.
A sex-linked recessive disorder with
retardation of growth, peculiar hair, and
focal cerebral and cerebellar degeneration.
Pediatr. 1962;29(1):764-79.
10. Danks DM, Campbell PE, Stevens BJ, et
al. Menkes's kinky hair syndrome. An
inherited defect in copper absorption with
widespread effects. Pediatr. 1972;50(2):
188-201.
11. Jankov RP, Boerkoel CF, Hellmann J, et
al. Lethal neonatal Menkes disease with
severe vasculopathy and fractures. Acta
Paediatr. 1998;87(12):1297-300.
392
Menkes Disease. M Barzegar, et al
12. Proud VK, Mussell HG, Kaler SG, et al.
Distinctive Menkes disease variant with
occipital horns: delineation of natural
history and clinical phenotype. Am J Med
Genet. 1996;65(1):44-51.
13. Grover WD, Johnson WC, Henkin RI.
Clinical and biochemical aspect of
Trichopoliodystrophy.
Ann
Neurol.
1979;5(1):65-71.
14. Whiting DA. Structural abnormalities of
the hair shaft. J Am Acad Dermatol.
1987;16(1 pt1):1-25
15. Bahi-Buisson N, Kaminska A, Nabbout R,
et al. Epilepsy in Menkes Disease:
Analysis of Clinical Stages. Epilepsia
2006;47(2):380-6.
16. Gu YU, Kodama H, Sato E, et al. Prenatal
diagnosis of Menkes disease by genetic
analysis and copper measurement. Brain
Dev. 2002;24(7): 715-8.
17. Gu YH, Kodama H, Shiga K, et al. A
survey of Japanese patients with Menkes
disease from 1990 to 2003: incidence and
early signs before typical symptomatic
onset, pointing the way earlier diagnosis. J
Inherit Metab Dis. 2005;28(4):473-8.
18. Munakata M, Sakamoto O, Kitamure T, et
al. The effects of copper-histidine therapy
on brain metabolism in patients with
Menkes disease: a proton magnetic
resonance spectroscopic study. Brain Dev.
2005;27(4):297-300.
You might also like
- Menkes Kinky Hair Disease (Menkes Syndrome) - A Case ReportDocument5 pagesMenkes Kinky Hair Disease (Menkes Syndrome) - A Case ReportTannov SiregarNo ratings yet
- Menkes Disease As A Differential Diagnosis of Child AbuseDocument3 pagesMenkes Disease As A Differential Diagnosis of Child AbuserickyferdianNo ratings yet
- 39 Srinivas EtalDocument3 pages39 Srinivas EtaleditorijmrhsNo ratings yet
- The Myth of Autism: How a Misunderstood Epidemic Is Destroying Our ChildrenFrom EverandThe Myth of Autism: How a Misunderstood Epidemic Is Destroying Our ChildrenRating: 2.5 out of 5 stars2.5/5 (8)
- Bilateral Ptosis Unusual Presentation Cerebral Arteriovenous MalformationDocument3 pagesBilateral Ptosis Unusual Presentation Cerebral Arteriovenous MalformationRin4lNo ratings yet
- Mitochondrial Respiratory Chain Disorder in a Filipino ChildDocument6 pagesMitochondrial Respiratory Chain Disorder in a Filipino Childraysa sepriaNo ratings yet
- Non-Cardiac Digeorge Syndrome in An Adolescent Girl With Epilepsy (A Case Report)Document6 pagesNon-Cardiac Digeorge Syndrome in An Adolescent Girl With Epilepsy (A Case Report)IJAR JOURNALNo ratings yet
- 52 Tripathy EtalDocument5 pages52 Tripathy EtaleditorijmrhsNo ratings yet
- Marinesco-Sjo Gren Syndrome - 2013Document5 pagesMarinesco-Sjo Gren Syndrome - 2013Irfan RazaNo ratings yet
- Androgenic Alopecia Finished ThesisDocument73 pagesAndrogenic Alopecia Finished ThesisAkshay R Aiyar100% (2)
- 439 2007 Article 362Document6 pages439 2007 Article 362Zinik EşanuNo ratings yet
- Dyke-Davidoff-Masson Syndrome Case ReportDocument3 pagesDyke-Davidoff-Masson Syndrome Case ReportMukernas Perdossi 2021No ratings yet
- Neurohistiocytosis: Two Cases of A Rare DiseaseDocument8 pagesNeurohistiocytosis: Two Cases of A Rare DiseaseIJAR JOURNALNo ratings yet
- Encefalopatía Por Kernicterus. Serie Clínica: Kernicterus (Bilirubin Encephalopathy) : Case ReportsDocument8 pagesEncefalopatía Por Kernicterus. Serie Clínica: Kernicterus (Bilirubin Encephalopathy) : Case ReportsCarlos Eduardo SotoNo ratings yet
- Neurology Multiple Choice Questions With Explanations: Volume IFrom EverandNeurology Multiple Choice Questions With Explanations: Volume IRating: 4 out of 5 stars4/5 (7)
- Subhyaloid Hemorrhage in Cerebral Malaria: Case ReportDocument4 pagesSubhyaloid Hemorrhage in Cerebral Malaria: Case ReportFaza KahfiNo ratings yet
- The Myth of Autism: How a Misunderstood Epidemic Is Destroying Our Children, Expanded and Revised EditionFrom EverandThe Myth of Autism: How a Misunderstood Epidemic Is Destroying Our Children, Expanded and Revised EditionRating: 2.5 out of 5 stars2.5/5 (3)
- Angelman syndrome and Rett syndrome genetic linkDocument6 pagesAngelman syndrome and Rett syndrome genetic linkAkmal NugrahaNo ratings yet
- A New Familial Adult-Onset Leukodystrophy Manifesting As Cerebellar Ataxia and DementiaDocument9 pagesA New Familial Adult-Onset Leukodystrophy Manifesting As Cerebellar Ataxia and DementiaKristineNo ratings yet
- Essential thrombocythaemia presenting as third nerve paralysisDocument2 pagesEssential thrombocythaemia presenting as third nerve paralysisMilutin MelkaNo ratings yet
- Juvenile Metachromatic Leucodystrophy A Rare Case ReportDocument2 pagesJuvenile Metachromatic Leucodystrophy A Rare Case ReportInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Clinical Reasoning: A Young Man With Recurrent Paralysis, Revisable White Matter Lesions and Peripheral Neuropathy Word Count: 945Document13 pagesClinical Reasoning: A Young Man With Recurrent Paralysis, Revisable White Matter Lesions and Peripheral Neuropathy Word Count: 945aileenzhongNo ratings yet
- Sinus Pericranii in Achondroplasia: A Case Report and Review of The LiteratureDocument4 pagesSinus Pericranii in Achondroplasia: A Case Report and Review of The Literaturechristian roblesNo ratings yet
- NurologyDocument3 pagesNurologytorosvartaniantorosNo ratings yet
- DDMSDocument5 pagesDDMSaghniajolandaNo ratings yet
- JNTRUnivHealthSci33195-154409 041720Document4 pagesJNTRUnivHealthSci33195-154409 041720chayankkuNo ratings yet
- Wooly Hair Palmoplantar Keratoderma: A Case ReportDocument3 pagesWooly Hair Palmoplantar Keratoderma: A Case ReportIJAR JOURNALNo ratings yet
- Cri Du ChatDocument16 pagesCri Du ChatJet LeeNo ratings yet
- 4 Epilepsy in A PatientDocument4 pages4 Epilepsy in A Patientarturschander3614No ratings yet
- Comment Letters: Similarity of Balloon Cells in Focal Cortical Dysplasia To Giant Cells in Tuberous SclerosisDocument4 pagesComment Letters: Similarity of Balloon Cells in Focal Cortical Dysplasia To Giant Cells in Tuberous SclerosisashfaqamarNo ratings yet
- Epilepsia EpicongressDocument245 pagesEpilepsia EpicongressВасилий КоптеловNo ratings yet
- Winawer Et Al-2018-Annals of NeurologyDocument14 pagesWinawer Et Al-2018-Annals of NeurologyAndoingNo ratings yet
- Cerebrotendinous-xanthomatosis;-a-genetic-conditioDocument5 pagesCerebrotendinous-xanthomatosis;-a-genetic-conditioangelluis1980No ratings yet
- BH 010060 ENGDocument2 pagesBH 010060 ENGCarlosNo ratings yet
- Rare CJD Presenting as Treatment Resistant DepressionDocument12 pagesRare CJD Presenting as Treatment Resistant DepressionpigzeniNo ratings yet
- Hypocalcemia - Diagnosis and Treatment - Endotext - NCBI BookshelfDocument31 pagesHypocalcemia - Diagnosis and Treatment - Endotext - NCBI Bookshelfgalnaresdaniela7No ratings yet
- Morphometry of WS On ASDocument6 pagesMorphometry of WS On ASAdrien DuthéNo ratings yet
- Ecr2013 C-2557Document33 pagesEcr2013 C-2557olaNo ratings yet
- Neurodegener RalucaDocument88 pagesNeurodegener RalucaAlexandra AnaellyNo ratings yet
- Hutchinson-Gilford Progeria Syndrome: Net CaseDocument4 pagesHutchinson-Gilford Progeria Syndrome: Net Casesario indriayaniNo ratings yet
- Pallister-Killian Mosaic Syndrome in An Omani NewbDocument5 pagesPallister-Killian Mosaic Syndrome in An Omani NewbFrank Harry LampardNo ratings yet
- Acute Disseminated Encephalomyelitis in Young Female - A Diagnosis of ExclusionDocument3 pagesAcute Disseminated Encephalomyelitis in Young Female - A Diagnosis of ExclusionIJAR JOURNALNo ratings yet
- Leukodystrophies and MucopolysacridosisDocument78 pagesLeukodystrophies and MucopolysacridosisMobin Ur Rehman KhanNo ratings yet
- Hemimegalencephaly Case Report Highlights Rare Brain ConditionDocument4 pagesHemimegalencephaly Case Report Highlights Rare Brain ConditionAnonymous FAyZgchPr6No ratings yet
- The Dangers of PRESDocument5 pagesThe Dangers of PRESShahnaaz ShahNo ratings yet
- Prion Diseases: Dinusha Yagama de Silva Group 11 Semester 7Document30 pagesPrion Diseases: Dinusha Yagama de Silva Group 11 Semester 7Tom perryNo ratings yet
- Congenitalmyopathies Andmusculardystrophies: Heather R. Gilbreath,, Diana Castro,, Susan T. IannacconeDocument15 pagesCongenitalmyopathies Andmusculardystrophies: Heather R. Gilbreath,, Diana Castro,, Susan T. IannacconeRizki Nandasari SulbahriNo ratings yet
- Ischemic Stroke in A 3-Year-Old Child Revealing Thrombosis of The Vertebrobasilar Territory, A Case ReportDocument5 pagesIschemic Stroke in A 3-Year-Old Child Revealing Thrombosis of The Vertebrobasilar Territory, A Case ReportIJAR JOURNALNo ratings yet
- ng00229 PDFDocument3 pagesng00229 PDFPaijo SusenoNo ratings yet
- Cerebral Palsy 2Document11 pagesCerebral Palsy 2waheedNo ratings yet
- Case Report: de Morsier Syndrome Associated With Periventricular Nodular HeterotopiaDocument4 pagesCase Report: de Morsier Syndrome Associated With Periventricular Nodular HeterotopiahypnonautNo ratings yet
- Le Mire 2004Document2 pagesLe Mire 2004Fapuw ParawansaNo ratings yet
- Subdural Hematoma Case PresentationDocument20 pagesSubdural Hematoma Case PresentationDarwin Arockia RajNo ratings yet
- Neuro PPT Format 3Document23 pagesNeuro PPT Format 3Deepak JhaNo ratings yet
- Case Report: Acute Ischemic Stroke Secondary To Iron Deficiency Anemia: A Case ReportDocument6 pagesCase Report: Acute Ischemic Stroke Secondary To Iron Deficiency Anemia: A Case ReportMaya QadrianiNo ratings yet
- Baraitser-Winter CerebrofrontofacialDocument7 pagesBaraitser-Winter Cerebrofrontofacialthaismartins023No ratings yet
- Clinical Reasoning: Rare Cause of Hemiparesis and Ataxia in A 36-Year-Old ManDocument8 pagesClinical Reasoning: Rare Cause of Hemiparesis and Ataxia in A 36-Year-Old MansamuelNo ratings yet
- MRI Findings in Susac SyndromeDocument6 pagesMRI Findings in Susac SyndromeLeonardoBubackNo ratings yet
- Hunter Syndrome A Case ReportDocument4 pagesHunter Syndrome A Case ReportResearch ParkNo ratings yet
- Disorders of Copper Homeostasis and HomoeopathyDocument13 pagesDisorders of Copper Homeostasis and HomoeopathyDr. Rajneesh Kumar Sharma MD Hom100% (1)
- LRR FMGE BiochemistryDocument226 pagesLRR FMGE BiochemistryVishal KumarNo ratings yet
- NEET PG 2019 Question Paper With SolutionsDocument364 pagesNEET PG 2019 Question Paper With SolutionsmedpoxNo ratings yet
- Sex Linked Inheritance ExplainedDocument37 pagesSex Linked Inheritance ExplainedJacNo ratings yet
- Oral Aspects of Metabolic Disorders 2167 0943 1000178Document4 pagesOral Aspects of Metabolic Disorders 2167 0943 1000178Kiki KikukNo ratings yet
- Copper in The Human Organism: Mini-ReviewDocument11 pagesCopper in The Human Organism: Mini-ReviewAwalliantoniNo ratings yet
- Trace Elements: Dr. Faryal Husnain PGR Clinical ChemistryDocument28 pagesTrace Elements: Dr. Faryal Husnain PGR Clinical ChemistryFaryalBalochNo ratings yet
- Sex Linked Inheritance Pedigree 2016Document37 pagesSex Linked Inheritance Pedigree 2016Krisburt Delos SantosNo ratings yet
- Globular Proteins: Dr. Sujin Bao, SJSMDocument27 pagesGlobular Proteins: Dr. Sujin Bao, SJSMethanNo ratings yet
- Urban Animal Trace KitDocument10 pagesUrban Animal Trace KitArghyadeep MitraNo ratings yet
- ATP7A Mutation DatabaseDocument5 pagesATP7A Mutation DatabaseanilmeherNo ratings yet
- NEET Jan 2019 PaperDocument364 pagesNEET Jan 2019 PaperDivya RagavanNo ratings yet
- Courses - Washington.edu - Gs361win - Problems - PS3-2 (Genome 361 Winter 2012)Document7 pagesCourses - Washington.edu - Gs361win - Problems - PS3-2 (Genome 361 Winter 2012)slee25No ratings yet
- CopperDocument110 pagesCopperVirra Mayang ArumNo ratings yet
- Hair Elements 2013Document5 pagesHair Elements 2013Meghanaram33No ratings yet
- Doctors Data - Result - 3287 PDFDocument7 pagesDoctors Data - Result - 3287 PDFAmine EmineNo ratings yet
- Early Treatment of Menkes DiseaseDocument11 pagesEarly Treatment of Menkes DiseaseMarco Miranda RodríguezNo ratings yet