Professional Documents
Culture Documents
Acute Glomerulonephritis: Background, Pathophysiology, Etiology
Uploaded by
'Riku' Pratiwie TunaCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Acute Glomerulonephritis: Background, Pathophysiology, Etiology
Uploaded by
'Riku' Pratiwie TunaCopyright:
Available Formats
Acute Glomerulonephritis: Background, Pathophysiology, Etiology
6/29/15, 9:49 AM
Acute Glomerulonephritis
Author: Malvinder S Parmar, MB, MS; Chief Editor: Vecihi Batuman, MD more...
Updated: Dec 12, 2014
Background
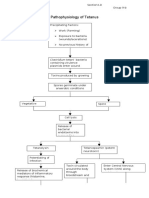
Acute glomerulonephritis (GN) comprises a specific set of renal diseases in which an immunologic mechanism
triggers inflammation and proliferation of glomerular tissue that can result in damage to the basement membrane,
mesangium, or capillary endothelium. Acute poststreptococcal glomerulonephritis (PSGN) is the archetype of
acute GN. Acute nephritic syndrome is the most serious and potentially devastating form of the various renal
syndromes.
Hippocrates originally described the natural history of acute GN, writing of back pain and hematuria followed by
oliguria or anuria. Richard Bright described acute GN clinically in 1827, which led to the eponymic designation
Bright disease. With the development of the microscope, Langhans was later able to describe these
pathophysiologic glomerular changes.
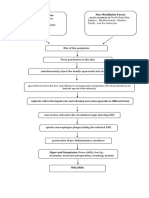
Acute GN is defined as the sudden onset of hematuria, proteinuria, and red blood cell (RBC) casts. This clinical
picture is often accompanied by hypertension, edema, azotemia (ie, decreased glomerular filtration rate [GFR]),
and renal salt and water retention. Acute GN can be due to a primary renal disease or to a systemic disease. Most
original research focuses on acute PSGN.
Treatment of PSGN is mainly supportive, because there is no specific therapy for renal disease. When acute GN is
associated with chronic infections, the underlying infections must be treated. This article addresses the aspects of
GN that are relevant to its acute management.
Go to Emergent Management of Acute Gldmerulonephritis and Acute Poststreptococcal Glomerulonephritis for
complete information on these topics.
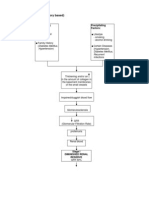
Pathophysiology
Glomerular lesions in acute GN are the result of glomerular deposition or in situ formation of immune complexes.
On gross appearance, the kidneys may be enlarged up to 50%. Histopathologic changes include swelling of the
glomerular tufts and infiltration with polymorphonucleocytes (see Histologic Findings). Immunofluorescence
reveals deposition of immunoglobulins and complement.
Except in PSGN, the exact triggers for the formation of the immune complexes are unclear. In PSGN, involvement
of derivatives of streptococcal proteins has been reported. A streptococcal neuraminidase may alter host
immunoglobulin G (IgG). IgG combines with host antibodies. IgG/anti-IgG immune complexes are formed and then
collect in the glomeruli. In addition, elevations of antibody titers to other antigens, such as antistreptolysin O or
antihyaluronidase, DNAase-B, and streptokinase, provide evidence of a recent streptococcal infection.
Structural and functional changes
Acute GN involves both structural changes and functional changes.
Structurally, cellular proliferation leads to an increase in the number of cells in the glomerular tuft because of the
proliferation of endothelial, mesangial,[1] and epithelial cells. The proliferation may be endocapillary (ie, within the
confines of the glomerular capillary tufts) or extracapillary (ie, in the Bowman space involving the epithelial cells).
In extracapillary proliferation, proliferation of parietal epithelial cells leads to the formation of crescents, a feature
characteristic of certain forms of rapidly progressive GN.
Leukocyte proliferation is indicated by the presence of neutrophils and monocytes within the glomerular capillary
lumen and often accompanies cellular proliferation.
Glomerular basement membrane thickening appears as thickening of capillary walls on light microscopy. On
electron microscopy, this may appear as the result of thickening of basement membrane proper (eg, diabetes) or
deposition of electron-dense material, either on the endothelial or epithelial side of the basement membrane.
Electron-dense deposits can be subendothelial, subepithelial, intramembranous, or mesangial, and they
correspond to an area of immune complex deposition.
Hyalinization or sclerosis indicates irreversible injury.
These structural changes can be focal, diffuse or segmental, or global.
Functional changes include proteinuria, hematuria, reduction in GFR (ie, oligoanuria), and active urine sediment
with RBCs and RBC casts. The decreased GFR and avid distal nephron salt and water retention result in
expansion of intravascular volume, edema, and, frequently, systemic hypertension.
Poststreptococcal glomerulonephritis
Streptococcal M-protein was previously believed to be responsible for PSGN, but the studies on which this belief
was based have been discounted. Nephritis-associated streptococcal cationic protease and its zymogen precursor
(nephritis-associated plasmin receptor [NAPlr]) have been identified as a glyceraldehyde-3-phosphate
dehydrogenase that functions as a plasmin(ogen) receptor.
Immunofluorescence staining of renal biopsy tissues with anti-NAPlr antibody revealed glomerular NAPlr
deposition in early-phase acute PSGN, and glomerular plasmin activity was almost identical to NAPlr deposition in
http://emedicine.medscape.com/article/239278-overview#showall
Page 1 of 5
Acute Glomerulonephritis: Background, Pathophysiology, Etiology
6/29/15, 9:49 AM
renal biopsy tissues of acute PSGN patients. These data suggest that NAPlr may contribute to the pathogenesis of
acute PSGN by maintaining plasmin activity.[2]
Antibody levels to nephritis-associated protease (NAPR) are elevated in streptococcal infections (group A, C, and
G) associated with GN but are not elevated in streptococcal infections without GN, whereas anti-streptolysin-O
titers are elevated in both circumstances. These antibodies to NAPR persist for years and perhaps are protective
against further episodes of PSGN. In a study in adults, the two most frequently identified infectious agents were
streptococci (27.9%) and staphylococci (24.4%).[3]
Go to Acute Poststreptococcal Glomerulonephritis for complete information on this topic.
Etiology
The causal factors that underlie acute GN can be broadly divided into infectious and noninfectious groups.
Infectious
The most common infectious cause of acute GN is infection by Streptococcus species (ie, group A, betahemolytic). Two types have been described, involving different serotypes:
Serotype 12 - Poststreptococcal nephritis due to an upper respiratory infection, occurring primarily in the
winter months
Serotype 49 - Poststreptococcal nephritis due to a skin infection, usually observed in the summer and fall
and more prevalent in southern regions of the United States
PSGN usually develops 1-3 weeks after acute infection with specific nephritogenic strains of group A betahemolytic streptococcus. The incidence of GN is approximately 5-10% in persons with pharyngitis and 25% in
those with skin infections.
Nonstreptococcal postinfectious GN may also result from infection by other bacteria, viruses, parasites, or fungi.
Bacteria besides group A streptococci that can cause acute GN include diplococci, other streptococci,
staphylococci, and mycobacteria. Salmonella typhosa, Brucella suis, Treponema pallidum, Corynebacterium bovis,
and actinobacilli have also been identified.
Cytomegalovirus (CMV), coxsackievirus, Epstein-Barr virus (EBV), hepatitis B virus (HBV),[4] rubella, rickettsiae
(as in scrub typhus), and mumps virus are accepted as viral causes only if it can be documented that a recent
group A beta-hemolytic streptococcal infection did not occur. Acute GN has been documented as a rare
complication of hepatitis A.[5]
Attributing glomerulonephritis to a parasitic or fungal etiology requires the exclusion of a streptococcal infection.
Identified organisms include Coccidioides immitis and the following parasites: Plasmodium malariae, Plasmodium
falciparum, Schistosoma mansoni, Toxoplasma gondii, filariasis, trichinosis, and trypanosomes.
Noninfectious
Noninfectious causes of acute GN may be divided into primary renal diseases, systemic diseases, and
miscellaneous conditions or agents.
Multisystem systemic diseases that can cause acute GN include the following:
Vasculitis (eg, granulomatosis with polyangiitis [Wegener granulomatosis]) - This causes glomerulonephritis
that combines upper and lower granulomatous nephritides).
Collagen-vascular diseases (eg, systemic lupus erythematosus [SLE]) This causes glomerulonephritis
through renal deposition of immune complexes).
Hypersensitivity vasculitis This encompasses a heterogeneous group of disorders featuring small vessel
and skin disease.
Cryoglobulinemia This causes abnormal quantities of cryoglobulin in plasma that result in repeated
episodes of widespread purpura and cutaneous ulcerations upon crystallization.
Polyarteritis nodosa - This causes nephritis from a vasculitis involving the renal arteries.
Henoch-Schnlein purpura This causes a generalized vasculitis resulting in glomerulonephritis.
Goodpasture syndrome This causes circulating antibodies to type IV collagen and often results in a
rapidly progressive oliguric renal failure (weeks to months).
Primary renal diseases that can cause acute GN include the following:
Membranoproliferative glomerulonephritis ( MPGN) - This is due to the expansion and proliferation of
mesangial cells as a consequence of the deposition of complements. Type I refers to the granular
deposition of C3; type II refers to an irregular process.
Berger disease (IgG-immunoglobulin A [IgA] nephropathy) - This causes GN as a result of diffuse
mesangial deposition of IgA and IgG.
Pure mesangial proliferative GN [1]
Idiopathic rapidly progressive glomerulonephritis - This form of GN is characterized by the presence of
glomerular crescents. Three types have been distinguished: Type I is an antiglomerular basement
membrane disease, type II is mediated by immune complexes, and type III is identified by antineutrophil
cytoplasmic antibody (ANCA).
Miscellaneous noninfectious causes of acute GN include the following:
Guillain-Barr syndrome
Irradiation of Wilms tumor
Diphtheria-pertussis-tetanus (DPT) vaccine
Serum sickness
Epidermal growth factor receptor activation, [6] and possibly its inhibition by cetuximab [7]
Epidemiology
http://emedicine.medscape.com/article/239278-overview#showall
Page 2 of 5
Acute Glomerulonephritis: Background, Pathophysiology, Etiology
6/29/15, 9:49 AM
United States statistics
GN represents 10-15% of glomerular diseases. Variable incidence has been reported, in part because of the
subclinical nature of the disease in more than half the affected population. Despite sporadic outbreaks, the
incidence of PSGN has fallen over the past few decades. Factors responsible for this decline may include better
health care delivery and improved socioeconomic conditions.
GN comprises 25-30% of all cases of end-stage renal disease (ESRD). About one fourth of patients present with
acute nephritic syndrome. Most cases that progress do so relatively quickly, and end-stage renal failure may occur
within weeks or months of the onset of acute nephritic syndrome. Asymptomatic episodes of PSGN exceed
symptomatic episodes by a ratio of 3-4:1.
International statistics
Worldwide, Berger disease is the most common cause of GN.
With some exceptions, the incidence of PSGN has fallen in most Western countries. PSGN remains much more
common in regions such as Africa, the Caribbean, India, Pakistan, Malaysia, Papua New Guinea, and South
America. In Port Harcourt, Nigeria, the incidence of acute GN in children aged 3-16 years was 15.5 cases per year,
with a male-to-female ratio of 1.1:1; the current incidence is not much different.[8]
Geographic and seasonal variations in the prevalence of PSGN are more marked for pharyngeally associated GN
than for cutaneously associated disease.[8, 9, 10]
Age-, sex-, and race-related demographics
Postinfectious GN can occur at any age but usually develops in children. Most cases occur in patients aged 5-15
years; only 10% occur in patients older than 40 years. Outbreaks of PSGN are common in children aged 6-10
years. Acute nephritis may occur at any age, including infancy.
Acute GN predominantly affects males (2:1 male-to-female ratio). Postinfectious GN has no predilection for any
racial or ethnic group. A higher incidence (related to poor hygiene) may be observed in some socioeconomic
groups.
Prognosis
Most epidemic cases follow a course ending in complete patient recovery (as many as 100%). The mortality of
acute GN in the most commonly affected age group, pediatric patients, has been reported at 0-7%.
Sporadic cases of acute nephritis often progress to a chronic form. This progression occurs in as many as 30% of
adult patients and 10% of pediatric patients. GN is the most common cause of chronic renal failure (25%).
In PSGN, the long-term prognosis generally is good. More than 98% of individuals are asymptomatic after 5 years,
with chronic renal failure reported 1-3% of the time.
Within a week or so of onset, most patients with PSGN begin to experience spontaneous resolution of fluid
retention and hypertension. C3 levels may normalize within 8 weeks after the first sign of PSGN. Proteinuria may
persist for 6 months and microscopic hematuria for up to 1 year after onset of nephritis.
Eventually, all urinary abnormalities should disappear, hypertension should subside, and renal function should
return to normal. In adults with PSGN, full recovery of renal function can be expected in just over half of patients,
and prognosis is dismal in patients with underlying diabetic glomerulosclerosis. Few patients with acute nephritis
develop rapidly progressive renal failure.
Approximately 15% of patients at 3 years and 2% of patients at 7-10 years may have persistent mild proteinuria.
Long-term prognosis is not necessarily benign. Some patients may develop hypertension, proteinuria, and renal
insufficiency as long as 10-40 years after the initial illness. Immunity to type M protein is type-specific, long-lasting,
and protective. Repeated episodes of PSGN are therefore unusual.
The prognosis for nonstreptococcal postinfectious GN depends on the underlying agent, which must be identified
and addressed. Generally, the prognosis is worse in patients with heavy proteinuria, severe hypertension, and
significant elevations of creatinine level. Nephritis associated with methicillin-resistant Staphylococcus aureus
(MRSA) and chronic infections usually resolves after treatment of the infection.
Other causes of acute GN have outcomes varying from complete recovery to complete renal failure. The prognosis
depends on the underlying disease and the overall health of the patient. The occurrence of cardiopulmonary or
neurologic complications worsens the prognosis.
Murakami and colleagues examined the clinical characteristics and pathological patterns of postinfectious
glomerulonephritis in 72 HIV-infected patients. The most common infectious agent was Staphylococcus. During a
median of 17 months of follow-up, pathological patterns had no significant effects on renal outcomes. Mortality
occurred in 14 patients overall, and mortality rates were significantly elevated among the 28 patients with healed
postinfectious glomerulonephritis.[11]
In a retrospective study of 101 patients with severe lupus and rapidly progressive glomerulonephritis and 200
lupus patient controls who were followed for a median of 4 years, rapidly progressive glomerulonephritis was
associated with poorer treatment response, atrophy and fibrosis, severe renal manifestations, serious sclerotic and
crescentic glomeruli lesions, severe tubulointerstitial inflammation, and prominent leukocyte infiltration. Serum
creatinine levels and the proportion of crescents were the most important predictors of developing end-stage renal
disease.[12]
Patient Education
Counsel patients about the need for the following measures:
Salt restriction during the acute phase to control edema and volume-related hypertension
http://emedicine.medscape.com/article/239278-overview#showall
Page 3 of 5
Acute Glomerulonephritis: Background, Pathophysiology, Etiology
6/29/15, 9:49 AM
Blood pressure monitoring at periodic intervals
Ongoing long-term monitoring of patients with persistent urinary abnormalities and elevated blood pressure
Consideration of protein restriction and angiotensin-converting enzyme (ACE) inhibitors (in patients who
show evidence of persistent abnormalities or in those who develop late evidence of progressive disease)
Early antibiotic treatment of close contacts
For patient education resources, see the Kidneys and Urinary System Center, as well as Blood in the Urine.
Contributor Information and Disclosures
Author
Malvinder S Parmar, MB, MS FRCP(C), FACP, FASN, Associate Professor, Department of Internal Medicine,
Northern Ontario School of Medicine; Assistant Professor, Department of Medicine, University of Ottawa Faculty
of Medicine; Consulting Physician, Timmins and District Hospital, Ontario, Canada
Malvinder S Parmar, MB, MS is a member of the following medical societies: American College of Physicians,
American Society of Nephrology, Canadian Medical Association, Ontario Medical Association, Royal College of
Physicians and Surgeons of Canada
Disclosure: Nothing to disclose.
Specialty Editor Board
Francisco Talavera, PharmD, PhD Adjunct Assistant Professor, University of Nebraska Medical Center
College of Pharmacy; Editor-in-Chief, Medscape Drug Reference
Disclosure: Received salary from Medscape for employment.
Ajay K Singh, MB, MRCP, MBA Associate Professor of Medicine, Harvard Medical School; Director of Dialysis,
Renal Division, Brigham and Women's Hospital; Director, Brigham/Falkner Dialysis Unit, Faulkner Hospital
Disclosure: Nothing to disclose.
Chief Editor
Vecihi Batuman, MD FACP, FASN, Huberwald Professor of Medicine, Section of Nephrology-Hypertension,
Tulane University School of Medicine; Chief, Renal Section, Southeast Louisiana Veterans Health Care System
Vecihi Batuman, MD is a member of the following medical societies: American College of Physicians, American
Society of Hypertension, American Society of Nephrology, International Society of Nephrology
Disclosure: Nothing to disclose.
Additional Contributors
Chike Magnus Nzerue, MD, FACP Professor of Medicine, Associate Dean for Clinical Affairs, Meharry Medical
College
Chike Magnus Nzerue, MD, FACP is a member of the following medical societies: American Association for the
Advancement of Science, American College of Physicians, American College of Physicians-American Society of
Internal Medicine, American Society of Nephrology, National Kidney Foundation
Disclosure: Nothing to disclose.
References
1. Wen YK, Chen ML. The significance of atypical morphology in the changes of spectrum of postinfectious
glomerulonephritis. Clin Nephrol. 2010 Mar. 73(3):173-9. [Medline].
2. Oda T, Yoshizawa N, Yamakami K, Sakurai Y, Takechi H, Yamamoto K, et al. The role of nephritisassociated plasmin receptor (NAPlr) in glomerulonephritis associated with streptococcal infection. J
Biomed Biotechnol. 2012. 2012:417675. [Medline]. [Full Text].
3. Nasr SH, Markowitz GS, Stokes MB, et al. Acute postinfectious glomerulonephritis in the modern era:
experience with 86 adults and review of the literature. Medicine (Baltimore). 2008 Jan. 87(1):21-32.
[Medline].
4. Safadi R, Almog Y, Dranitzki-Elhalel M, Rosenmann E, Tur-Kaspa R. Glomerulonephritis associated with
acute hepatitis B. Am J Gastroenterol. 1996 Jan. 91(1):138-9. [Medline].
5. Aggarwal A, Kumar D, Kumar R. Acute glomerulonephritis in hepatitis A virus infection: a rare
presentation. Trop Doct. 2009 Jul. 39(3):186-7. [Medline].
6. Tang J, Liu N, Zhuang S. Role of epidermal growth factor receptor in acute and chronic kidney injury.
Kidney Int. 2013 Jan 16. [Medline].
7. Sasaki K, Anderson E, Shankland SJ, Nicosia RF. Diffuse Proliferative Glomerulonephritis Associated
With Cetuximab, an Epidermal Growth Factor Receptor Inhibitor. Am J Kidney Dis. 2013 Mar 6. [Medline].
8. Anochie I, Eke F, Okpere A. Childhood acute glomerulonephritis in Port Harcourt, Rivers State, Nigeria.
Niger J Med. 2009 Apr-Jun. 18(2):162-7. [Medline].
9. Wong W, Morris MC, Zwi J. Outcome of severe acute post-streptococcal glomerulonephritis in New
Zealand children. Pediatr Nephrol. 2009 May. 24(5):1021-6. [Medline].
10. Becquet O, Pasche J, Gatti H, et al. Acute post-streptococcal glomerulonephritis in children of French
Polynesia: a 3-year retrospective study. Pediatr Nephrol. 2010 Feb. 25(2):275-80. [Medline].
11. Murakami CA, Attia D, Carter-Monroe N, Lucas GM, Estrella MM, Fine DM, et al. The clinical
characteristics and pathological patterns of postinfectious glomerulonephritis in HIV-infected patients.
PLoS One. 2014. 9(10):e108398. [Medline]. [Full Text].
http://emedicine.medscape.com/article/239278-overview#showall
Page 4 of 5
Acute Glomerulonephritis: Background, Pathophysiology, Etiology
6/29/15, 9:49 AM
12. Chen S, Chen H, Liu Z, Zhang H, Hu W, Tang Z, et al. Pathological spectrums and renal prognosis of
severe lupus patients with rapidly progressive glomerulonephritis. Rheumatol Int. 2014 Oct 4. [Medline].
13. Nebuloni M, Barbiano di Belgiojoso G, Genderini A, et al. Glomerular lesions in HIV-positive patients: a
20-year biopsy experience from Northern Italy. Clin Nephrol. 2009 Jul. 72(1):38-45. [Medline].
Medscape Reference 2011 WebMD, LLC
http://emedicine.medscape.com/article/239278-overview#showall
Page 5 of 5
You might also like
- Pathophysiology of TuberculosisDocument2 pagesPathophysiology of TuberculosisLeikkaNo ratings yet
- Obstructive Jaundice, Choledocholithiasis Calculus CholecystitisDocument36 pagesObstructive Jaundice, Choledocholithiasis Calculus CholecystitisJohn Philip M. Lacas RN100% (2)
- Case Study in KidneyDocument3 pagesCase Study in KidneyVenice VelascoNo ratings yet
- Rabies Case PresentationDocument36 pagesRabies Case PresentationViorica Gavriliță100% (1)
- BFCDocument8 pagesBFCIrene GunongNo ratings yet
- Acute Post-Streptococcal GlomerulonephritisDocument15 pagesAcute Post-Streptococcal GlomerulonephritisJeanne Marie ValesNo ratings yet
- Nursing Care Plan for Crohn's Disease ManagementDocument13 pagesNursing Care Plan for Crohn's Disease ManagementJay Jay JayyiNo ratings yet
- Patho DengueDocument3 pagesPatho DengueLindy Shane BoncalesNo ratings yet
- Course in The WardDocument4 pagesCourse in The Wardenzomarfal100% (1)
- Concept MapDocument1 pageConcept Mapadriana100% (1)
- Hypertension: Colegio de San Juan de LetranDocument13 pagesHypertension: Colegio de San Juan de LetranJenna AbuanNo ratings yet
- Drug Study: Sulodexide, Cilostazol, Metoprolol, Albumin, FurosemideDocument10 pagesDrug Study: Sulodexide, Cilostazol, Metoprolol, Albumin, FurosemideMei PayumoNo ratings yet
- Case Study ReportDocument23 pagesCase Study Reportapi-290866384No ratings yet
- Group 5 - Experiment No.10 - Culture and SensitivityDocument11 pagesGroup 5 - Experiment No.10 - Culture and SensitivityPMG BrightNo ratings yet
- Case Study 1 (Pneumonia)Document13 pagesCase Study 1 (Pneumonia)Kate EscotonNo ratings yet
- Pediatric Community Acquired Pneumonia - Moderate Risk: University of The Cordilleras College of NursingDocument22 pagesPediatric Community Acquired Pneumonia - Moderate Risk: University of The Cordilleras College of NursingJemimah AdaclogNo ratings yet
- Pathophysiology of Covid-19 Infection: What Is The Novel Coronavirus (Sars-Cov-2) Doing To Body? A Comprehensive Systematic ReviewDocument15 pagesPathophysiology of Covid-19 Infection: What Is The Novel Coronavirus (Sars-Cov-2) Doing To Body? A Comprehensive Systematic ReviewMade DeanaNo ratings yet
- Ulcerative ColitisDocument18 pagesUlcerative ColitisHoussein EL HajjNo ratings yet
- Age NCPDocument4 pagesAge NCPnj_pink081794No ratings yet
- Amoebiasis PathophysiologyDocument3 pagesAmoebiasis PathophysiologyApril CornejoNo ratings yet
- Case Report No1Document9 pagesCase Report No1Menn PetchuayNo ratings yet
- Cs AGNDocument177 pagesCs AGNMa Rafaela Rosales PalomponNo ratings yet
- Upper Gastrointestinal BleedingDocument69 pagesUpper Gastrointestinal Bleedingeliza luisNo ratings yet
- BSN4D-SG2 DM Type2Document201 pagesBSN4D-SG2 DM Type2Charisse CaydanNo ratings yet
- Nursing DiagnosisDocument5 pagesNursing DiagnosisGeovanni Rai HermanoNo ratings yet
- Part 1Document37 pagesPart 1anon_154149519No ratings yet
- Hypertension Pathophysiology and Treatment PDFDocument6 pagesHypertension Pathophysiology and Treatment PDFBella TogasNo ratings yet
- Acute Glomerulonephritis: What is it and What Causes itDocument12 pagesAcute Glomerulonephritis: What is it and What Causes itSara Sonnya Ayutthaya NapitupuluNo ratings yet
- Pathophysiology of Rheumatic Heart Disease to CardiomegalyDocument2 pagesPathophysiology of Rheumatic Heart Disease to CardiomegalyRj Avila100% (1)
- FilariasisDocument2 pagesFilariasismariaNo ratings yet
- Rabies: A Deadly Viral Disease Spread by Animal BitesDocument3 pagesRabies: A Deadly Viral Disease Spread by Animal BitesDavid CalaloNo ratings yet
- BioethicsCasesEEI 316232215 PDFDocument38 pagesBioethicsCasesEEI 316232215 PDFAman UllahNo ratings yet
- SEPTICARTHRITISDocument2 pagesSEPTICARTHRITISapi-3822433No ratings yet
- Nursing Care Plans for Pneumonia and HemorrhoidsDocument6 pagesNursing Care Plans for Pneumonia and HemorrhoidsCharlene Grace ReginoNo ratings yet
- CHF Group 3 Ncmb312 RleDocument39 pagesCHF Group 3 Ncmb312 RleMaica Lectana50% (2)
- Pneumonia Drug StudyDocument3 pagesPneumonia Drug Studyatienza02No ratings yet
- Dengue PathophysiologyDocument1 pageDengue PathophysiologyRafael Miguel Alon Protacio50% (2)
- Pathophysiology of Tetanus: Clostridium Tetani BacteriaDocument3 pagesPathophysiology of Tetanus: Clostridium Tetani BacteriaSlepy chng100% (1)
- Non-Modifiable Factor Modifiable Factor: South-East Asia, Eastern, Mediterranean, Western Pacific, and The AmericasDocument2 pagesNon-Modifiable Factor Modifiable Factor: South-East Asia, Eastern, Mediterranean, Western Pacific, and The Americaschristian quiaoitNo ratings yet
- NURSING CARE PLAN FOR RHEUMATIC HEART DISEASEDocument11 pagesNURSING CARE PLAN FOR RHEUMATIC HEART DISEASECharm TanyaNo ratings yet
- Relationship Between CKD Stage and Pulmonary Edema on Chest X-RayDocument6 pagesRelationship Between CKD Stage and Pulmonary Edema on Chest X-RayAnnisa RabbaniNo ratings yet
- Pathophysiology of HyperthyroidismDocument4 pagesPathophysiology of HyperthyroidismKitty YuffieNo ratings yet
- Facebook data privacy scandal affects 87 millionDocument1 pageFacebook data privacy scandal affects 87 millionAnne DSNo ratings yet
- Parenteral Fluid Therapy: Types of Intravenous SolutionDocument18 pagesParenteral Fluid Therapy: Types of Intravenous SolutionKathleen Joy Costales Magtanong100% (1)
- REVALIDADocument53 pagesREVALIDAMercy Anne EcatNo ratings yet
- B NCP On and Off Fever 2b ImproveddocxDocument6 pagesB NCP On and Off Fever 2b ImproveddocxKylie CatralNo ratings yet
- CholelithiasisDocument6 pagesCholelithiasismarkzamNo ratings yet
- AGE PathophysiologyDocument2 pagesAGE Pathophysiologyjosephcanlas67% (3)
- Dnrle Influenza NCPDocument3 pagesDnrle Influenza NCPEna RodasNo ratings yet
- Chronic Renal FailureDocument3 pagesChronic Renal FailureAura Salve Ildefonso AllasNo ratings yet
- College of Nursing: Cebu Normal UniversityDocument4 pagesCollege of Nursing: Cebu Normal UniversityFaye Andrea FranciscoNo ratings yet
- ParacetamolDocument2 pagesParacetamolsleep whatNo ratings yet
- The Pathophysiology of LabyrinthitisDocument2 pagesThe Pathophysiology of LabyrinthitisSurya Michael ChanceNo ratings yet
- Pathophysiology of Urinary Tract ObstructionDocument50 pagesPathophysiology of Urinary Tract ObstructionPryo UtamaNo ratings yet
- Tatz Pa ToolDocument23 pagesTatz Pa Toolian_mendoza_3No ratings yet
- PUCAN, Julienne BSN III-D - SGD - HYPO&HYPERCHLOREMIADocument10 pagesPUCAN, Julienne BSN III-D - SGD - HYPO&HYPERCHLOREMIAJulienne PucanNo ratings yet
- Nursing Care PlanDocument8 pagesNursing Care PlanVincent QuitorianoNo ratings yet
- Nephrithic Sydrome PDFDocument20 pagesNephrithic Sydrome PDFLeónGarcíaNo ratings yet
- Acute Glomerulonephritis: Causes, Symptoms and TreatmentDocument16 pagesAcute Glomerulonephritis: Causes, Symptoms and TreatmentPrabha Amandari SutyandiNo ratings yet
- Acute GlomerulonephritisDocument12 pagesAcute Glomerulonephritiskuchaibaru90No ratings yet
- Antihypertensive Drugs - Classification and SynthesisDocument14 pagesAntihypertensive Drugs - Classification and SynthesisCường NguyễnNo ratings yet
- High Risk New BornDocument36 pagesHigh Risk New BornIze C VijiNo ratings yet
- COPD A Multifactorial Systemic DiseaseDocument9 pagesCOPD A Multifactorial Systemic DiseaseNjala SankhulaniNo ratings yet
- CV Disease Drug StudyDocument11 pagesCV Disease Drug StudyMaria Francheska OsiNo ratings yet
- Angiotensin II Receptor AntagonistDocument22 pagesAngiotensin II Receptor AntagonistaakshitNo ratings yet
- Pelvic Floor Distress Inventory Questionnaire - Short Form 20Document2 pagesPelvic Floor Distress Inventory Questionnaire - Short Form 20Dana Ysabelle Ibarra100% (1)
- English Role Play Script for Nursing StudentsDocument4 pagesEnglish Role Play Script for Nursing StudentsIefana Fihtreey TaezZkeyaNo ratings yet
- Stillbirth FinalDocument21 pagesStillbirth Finalzahirah nurNo ratings yet
- Guide to Calcium, Phosphate, and Parathyroid Hormone DisordersDocument7 pagesGuide to Calcium, Phosphate, and Parathyroid Hormone DisordersGregNo ratings yet
- Nursing Care Plan Guide for Holistic Patient CareDocument23 pagesNursing Care Plan Guide for Holistic Patient CareRej PanganibanNo ratings yet
- Efficacy and Safety of Rabeprazole in Children.20Document10 pagesEfficacy and Safety of Rabeprazole in Children.20Ismy HoiriyahNo ratings yet
- Lecture - 3 Properties of Cardiac MuscleDocument35 pagesLecture - 3 Properties of Cardiac MuscleMRM7MDNo ratings yet
- PD2Document15 pagesPD2DrShobhit RajNo ratings yet
- Tugas Remedial PaperDocument7 pagesTugas Remedial PaperFujiNo ratings yet
- Tips For The NDECCDocument2 pagesTips For The NDECCMagda Jakubowska-EwiczNo ratings yet
- Makalah Basic Life SupportDocument22 pagesMakalah Basic Life SupportKustian PramuditaNo ratings yet
- Novus Knowledge Webinar - Feed Milling For Sustainable Food Production - Feed Mill Biosecurity - Dr. Charles Stark PDFDocument43 pagesNovus Knowledge Webinar - Feed Milling For Sustainable Food Production - Feed Mill Biosecurity - Dr. Charles Stark PDFaradhya bhatiya0% (1)
- Literature ReviewDocument3 pagesLiterature Reviewapi-534368223No ratings yet
- Hyponatremic Dehydration PathoDocument4 pagesHyponatremic Dehydration PathoENo ratings yet
- Discharge PlanningDocument1 pageDischarge PlanningChyNo ratings yet
- Table 5. Mrs. L's Braden ScoreDocument1 pageTable 5. Mrs. L's Braden ScoreEfryl MaeNo ratings yet
- DYSTOCIA TITLEDocument14 pagesDYSTOCIA TITLEsailor MoonNo ratings yet
- Nursing Responsibilities Adverse Effect Indication / Contraindication Mechanism of Action Drug Name IndicationDocument1 pageNursing Responsibilities Adverse Effect Indication / Contraindication Mechanism of Action Drug Name IndicationOmar IzzoNo ratings yet
- Promoting Hygiene Through Complete BathingDocument12 pagesPromoting Hygiene Through Complete BathingErlangga PratamaNo ratings yet
- Drug Name Mecahnism of Action Indication Side Effects Nursing Responsibilities Generic Name: Brand Name: Classification: - Body As A Whole: - DuringDocument2 pagesDrug Name Mecahnism of Action Indication Side Effects Nursing Responsibilities Generic Name: Brand Name: Classification: - Body As A Whole: - DuringhahahaNo ratings yet
- Cytomegalovirus in Primary ImmunodeficiencyDocument9 pagesCytomegalovirus in Primary ImmunodeficiencyAle Pushoa UlloaNo ratings yet
- Implant Surgery Complications - Etiology and TreatmentDocument10 pagesImplant Surgery Complications - Etiology and TreatmentStephanie JaramilloNo ratings yet
- 50 Prometric MCQ For GPDocument10 pages50 Prometric MCQ For GPDrkhslid8971% (7)
- 1 Quarter Examination in Tle 7Document3 pages1 Quarter Examination in Tle 7MARK FERNANDEZNo ratings yet
- E Diabetes CareDocument48 pagesE Diabetes CareRuwan ManjulaNo ratings yet