Professional Documents
Culture Documents
Notes Drying
Uploaded by
Peter Paul BucsitCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Notes Drying
Uploaded by
Peter Paul BucsitCopyright:
Available Formats
CHEMICAL ENGINEERING SERIES
DRYING
Compilation of Lectures and Solved Problems
CHEMICAL ENGINEERING SERIES 2
DRYING
DRYING
-
is the removal of relatively small amounts of solvent, at temperatures
below its boiling point, by circulating air or some other gas over the
material in order to carry away the solvent vapor.
This is an adiabatic (constant enthalpy) drying process in which heat
required for the vaporization of solvent comes solely from the sensible
heat of the frying medium
In the usual drying or dehumidification process, water is the solvent and
air is the drying medium. The drying process cools the air adiabatically at
a constant wet bulb. The dry bulb temperature approaches the wet bulb
temperature and could reach it at the saturation point.
Moisture Content, wet basis,
Expressed as kg moisture per kg wet solid or kg moisture per combined kg of
dry solid and moisture.
Moisture Content, dry basis,
Expressed as kg moisture per kg dry solid
Bound Moisture
Is the moisture content of a substance which exerts an equilibrium vapor
pressure less than that of the pure liquid at the same temperature; it is the
moisture difficult to remove, but which can be removed only under special
conditions
Unbound Moisture
Refers to the moisture content of a substance which exerts an equilibrium
vapor pressure equal to that of the pure liquid at the same temperature.
Equilibrium Moisture Content,
Xe
Is the limiting moisture to which a given material can be dried under specific
conditions of air temperature and humidity; corresponds to bound moisture
Free Moisture Content,
X X e
CHEMICAL ENGINEERING SERIES 3
DRYING
Moisture content of a substance in excess of the equilibrium moisture; only
free moisture can be evaporated, and the free moisture content of a solid
depends upon the vapor concentration in the gas
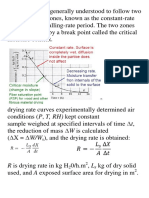
Critical Moisture Content,
Xc
The average moisture content at the end of constant rate drying period or at
the start of the falling rate period
Constant Rate Drying Period
The drying period during which the rate of water removal per unit of drying
surface is constant
Falling Rate Drying Period
The drying period during which the instantaneous drying rate continually
decreases
METHODSOF DRYING OPERATIONS
1. Batch Drying actually a semi-batch process wherein a quantity of the
substance to be dried is exposed to a continuously flowing stream of air
into which the moisture evaporates; batch or semi-batch equipment is
operated intermittently or cyclically under steady state conditions: the
dryer is charged with the substance, which remains in the equipment until
dry, whereupon the dryer is emptied and recharged with a fresh batch
2. Continuous Drying the substance to be dried as well as the gas passes
continually through the equipment; no typically stagewise methods are
ordinarily used, and all operations involve continuous contact of the gas
and the drying substance; continuous dryers are usually operated in
steady state fashion
METHODS OF SUPPLYING THE HEAT NECESSARY FOR EVAPORATION
OF MOISTURE
1. Direct Dryers heat is supplied entirely by direct contact of the
substance with the hot gas into which the evaporation takes place
2. Indirect Dryers heat is supplied quite independently of the gas used to
carry away the vaporized moisture
CHEMICAL ENGINEERING SERIES 4
DRYING
NATURE OF THE SUBSTANCE TO BE DRIED
1.
2.
3.
4.
Rigid Solid wood or fibreboard
Flexible material cloth or paper
Granular solid mass of crystals
Thick paste or thin slurry or a solution
HEAT TRANSFER IN DRYERS
CALCULATION OF HEAT DUTY:
Heat must be applied to a dryer to accomplish the following:
1. Heat the feed (solids and liquid) to the vaporization temperature
ps ( T v T si ) + X i C pL ( T v T si )
q1 =C
2. Vaporize the liquid
q 2=( X i X f ) v
3. Heat the product (solids and liquid) to their final temperature
ps ( T sf T v ) + X f C pL ( T sf T v )
q3 =C
4. Heat the vapour to its final temperature
q 4=( X f X i ) C pv ( T vf T v )
5. Heat the air or other added gas to final temperature
The total heat transferred per unit mass of dry bone solid is:
qT =q1 +q2 +q 3 +q 4
pv ( T vf T
qT =[ C ps ( T v T si ) + X i C pL ( T v T si ) ]+ [ ( X i X f ) v ] + [ C ps ( T sf T v ) + X f C pL ( T sf T v ) ] + [ ( X f X i ) C
qT
pv ( T vf T v ) ]+ [ ( X i X f ) v ]
= C ps ( T sf T si )+ C pL [ X i ( T v T si ) + X f ( T sf T v ) ] + [ ( X f X i ) C
s
m
In an adiabatic dryer, the heat transferred to the solids, liquid and vapour,
comes from the cooling of the gas
C si ( T hi T hf )
qT =W
HEAT TRANSFER COEFFICIENTS
CHEMICAL ENGINEERING SERIES 5
DRYING
q=UA T
qT =U a V T
EVALUATION OF HEAT TRANSFER COEFFICIENT
1. For air flowing parallel to the drying surface
Nu=
hy D e
=0.037 0.8 Pr 0.33
k
2. For flow of gas perpendicular to the surface, (air velocities between 0.90
and 4.5 m/s)
h y =24.2G0.37
(h y
BTU
lb
; G 2 )
ft h F
ft h
2
Constant drying rate is simply,
Rc =
m
v h y ( T T i )
=
A
i
HEAT TRANSFER UNITS
Some adiabatic dryers, especially rotary dryers, are conveniently rated in
terms of the number of heat transfer units they contain.
T hi
N t =
T hf
Nt=
d Th
T hT s
T hi T hf
When the initial liquid content of the solids is high and most of the heat
transferred is for vaporization,
may be taken as the logarithmic mean
difference between the dry bulb and wet bulb temperatures
CHEMICAL ENGINEERING SERIES 6
DRYING
= T
L= (
T
T hi T wi )( T hf T wf )
ln
( T hiT wi )
( T hf T wf )
T wi=T wf
= T
L=
T
ln
Nt=
ln
T hi T hf
( T hiT wi )
( T hf T wf )
T hi T hf
( T hiT wi )
=ln
T hi T hf
( T hf T wf )
( T hiT wi )
( T hf T wf )
T v =T wi
where:
qT
q
= total heat transferred per unit mass of dry bone solid
= rate of heat transfer in a section of the dryer
C ps
= specific heat of dry bone solid
C pL
= specific heat of liquid
C pv
= specific heat of vapour
C si
= humid heat of gas at inlet humidity
T si = temperature of feed
T sf
= final solids temperature
Tv
= vaporization temperature
T vf
= final vapour temperature
= heat of vaporization
CHEMICAL ENGINEERING SERIES 7
DRYING
m
s
= mass rate of dry bone solid
m
g
= mass rate of dry gas
= over-all heat transfer coefficient
= heat transfer area
average
temperature
difference
(not
necessarily
the
logarithmic mean)
= dryer volume
Ua
= volumetric heat transfer coefficient
Nu
= Nusselt number
hy
= heat transfer coefficient between gas and surface of slab
De
= equivalent diameter
= thermal conductivity
BATCH DRYING: CALCULATIONS OF DRYING TIME
Drying in batches is relatively an expensive operation and is consequently
limited to small-scale operations, to pilot plant and development work and
to drying valuable materials whose total cost will be little influenced by
added expense in the drying operations
Examples of batch dryers
o Tray, Cabinet or Shelf Dryers used for drying solids which must be
supported on trays
Unless stated otherwise, moisture contents of solids are on the wet basis
and should be converted to dry basis before solving any problem
Batch dryers operate under constant drying conditions
TIME OF DRYING UNDER CONSTANT DRYING CONDITIONS
CHEMICAL ENGINEERING SERIES 8
DRYING
Where:
= rate of drying, lb H2O/ft2h or kg H2O/m2h
Rf
= rate of drying at falling rate
Rc
= rate of drying at constant rate
Xe
= equilibrium moisture content (dry basis)
Xc
= critical moisture content (dry basis)
Xf
= final moisture content (dry basis)
Xi
= initial moisture content (dry basis)
= drying time
ms
= weight of dry bone solid, kg or lb
= total drying area, ft2 or m2
At Constant Drying Conditions (CDC)
R=
ms dX
A d
1. Constant Rate Period as long as the liquid covers the entire surface of
the solid, the rate of drying is constant. During this period, water diffuses
through the solid at a rate sufficient to keep the entire surface wet
R=
ms dX
A d
CHEMICAL ENGINEERING SERIES 9
DRYING
R=R c
c
Xc
ms
R d=
dX
A
0
X
i
c =
c =
ms
( X X i )
A Rc c
ms ( X i X c )
A Rc
2. Falling Rate Period when part of the solid surface is no longer wetted
by the liquid, the drying rate decreases. Most of the water escapes by
vaporizing at the surface of the solid
R ( X X e )
R=k ( X X e )
R=
ms dX
A d
k ( X X e )=
f
ms dX
A d
Xf
ms
dX
k d=
A X X X e
0
c
f =
k=
ms X f X e
ln
Ak
X c X e
Rc
X c X e
f =
ms ( X c X e )
X X e
ln f
A Rc
X c X e
CHEMICAL ENGINEERING SERIES 10
DRYING
f =
m s ( X c X e )
X c X e
ln
A Rc
X f Xe
3. Total Drying Time (Constant Rate + Falling Rate)
T =c +f
T =
ms ( X iX c ) ms ( X c X e )
X Xe
+
ln c
A Rc
A Rc
X f Xe
T =
ms
X X e
X i X c + ( X c X e ) ln c
A Rc
X f X e
)]
DRYING EQUIPMENT
1. Dryers for Solids and Pastes
a. Tray Dryers
Consists of a rectangular chamber of sheet metal containing two
trucks that supports racks; each rack carries a number of shallow
trays that are loaded with the material to be dried
Heated is circulated at 2 5 m/s between trays by fan and motor
and passes over heaters; air is distributed uniformly over the stack
of trays through baffles
Useful on small production rate; they find most frequent application
for valuable products like dyes and pharmaceuticals
b. Screen Conveyor Dryers
A layer (25 mm to 150 mm) thick of material to be dried is slowly
carried on a travelling metal screen through a long drying chamber
or tunnel
The chamber consists of series of separate sections, each with its
own fan and air heater. At the inlet end of the dryer, the air usually
passes upward through the screen and the solids; near the
discharge end, where the material is dry and may be dusty, air is
passed downward through the screen. The air temperature and
CHEMICAL ENGINEERING SERIES 11
DRYING
humidity may differ in the various sections to give optimum
conditions for drying at each point
Typically 2 m wide and 4 50 m long, giving drying times of 5 120
minutes; the minimum screen size is about 30 mesh
Handles variety of solids continuously and with a very gentle
action; particularly applicable when the drying conditions must be
appreciably changed as the moisture content of the solid is reduced
c. Tower dryers
Contains a series of circular trays mounted one above the other on
a central rotating shaft
Solid feed is dropped on the topmost tray is exposed to a stream of
hot air or gas that passes across the tray. The solid is then
scrapped off and dropped to the tray below. The flow of solids and
gas may be either parallel or counter-current
d. Rotary Dryers
Consists of a revolving cylindrical shell, horizontal or slightly
inclined toward the outlet
Wet feed enters one end of the cylinder; dry material discharges
from the other
Rotary dryers are heated by direct contact of gas with the solids, by
hot gas passing through an external jacket, or by steam condensing
in a set of longitudinal tubes mounted on the inner surface of the
shell
The allowable mass velocity of the gas in a direct dryer depends on
the dusting characteristics of the solid being dried and ranges from
2,000 to 25,000 kg/m2h for coarse particles; inlet gas temperatures
are typically 120 175C for steam heated air and 550 - 800C for
flue gas from a furnace.
Dryer diameters range from 1 3 m; the peripheral speed of the
shell is commonly 20 25 m/min.
Direct contact rotary dryers are designed on the basis of heat
transfer
qT =
0.5 G0.67
V T =0.125 DL G0.67 T
D
Ua=
0.5G0.67
D
Where:
qT
= rate of heat transfer, BTU/h
= dryer volume, ft3
CHEMICAL ENGINEERING SERIES 12
DRYING
L
T
= dryer length, ft
= average temperature difference, taken as logarithmic
mean of wet-blub depressions at inlet and outlet of the dryers
= mass velovity, lb/ft2h
= dryer diameter, ft
Ua
= volumetric heat transfer coefficient, BTU/ft3hF
e. Screw Conveyor Dryers
A continuous indirect-heat dryer, consisting essentially of a
horizontal screw conveyor (or paddle conveyor) enclosed in a
cylindrical jacketed shell
Solid fed in one end is conveyed slowly through the heated zone
and discharges from the other end.
The vapour evolved is
withdrawn through pipes set in the roof of the shell
Handles solids that are too fine and too sticky for rotary dryers;
they are completely enclosed and permit recovery of solvent
vapors with little or no dilution by air.
f. Fluid Bed Dryers
Solid particles are fluidized by air or gas in a boiling-bed unit;
mixing and heat transfer are very rapid; wet feed is admitted to the
top of the bed; dry product is taken out from the side, near the
bottom
ho D p
D G
=2.0+0.60 p
kf
f
0.50
C p f
kf
1/ 3
( ) ( )
Where:
ho
= heat transfer coefficient between and gas and solid,
BTU/ft2hF
Dp
= particle diameter, ft
kf
= thermal conductivity at mean film temperature,
BTU/fthF
g. Flash Dryers
Wet pulverized solid is transported for a few seconds in a hot gas
stream
CHEMICAL ENGINEERING SERIES 13
DRYING
The rate of heat transfer from the gas to the suspended solid
particles is high and drying is rapid so that no more than 3 or 4 s is
required to evaporate substantially all the moisture from the solid
Flash drying may be applied to sensitive materials that in other
dryers would have to be dried indirectly by a much cooler heating
medium
2. Dryers for Solutions and Slurries
a. Spray Dryers
A slurry or liquid solution is dispersed into a stream of hot gas in
the form of a mist of fine droplets. Moisture is rapidly vaporized
from the droplets, leaving residual particles of dry solid, which are
then separated from the gas stream. The flow of liquid and gas may
be co-current, counter current or a combination of both in the same
unit
Droplets are formed inside a cylindrical drying chamber by pressure
nozzles, two-fluid nozzles, or, in large dryers, high speed spray
disks
An equation for the volume-surface mean diameter
s
D
of the
drops from a disk atomizer is:
s
D
=0.4
r
L n r2
0.6
0.2
)( )(
L L p
2
0.1
Where:
s
D
= average drop diameter, m or ft
= disk radius, m or ft
= spray mass rate per unit length of disk periphery, kg/ms
or lb/fts
= surface tension of liquid, kg/m 3 or lb/ft3
= disk speed, r/s
= viscosity of liquid, Pas or lb/fts
Lp
= disk periphery, 2r, m or ft
b. Thin Film Dryers
Competitive with spray dryers but relatively expensive
c. Drum Dryers
CHEMICAL ENGINEERING SERIES 14
DRYING
Consist of one or more heated metal rolls on the outside of which a
thin layer of liquid is evaporated to dryness. Dried solid is scraped
off the rolls as they slowly revolve
You might also like
- Multiple choice questions on mechanics, heat and thermodynamicsDocument21 pagesMultiple choice questions on mechanics, heat and thermodynamicsGodwinNo ratings yet
- Liquid-Liquid Extraction Processes and EquilibriumDocument52 pagesLiquid-Liquid Extraction Processes and EquilibriumThelunatic ModNo ratings yet
- 2011 Trial Physics AnswersDocument27 pages2011 Trial Physics AnswersGeorgeNo ratings yet
- Drying 2 Class Notes PDFDocument18 pagesDrying 2 Class Notes PDFFarouk Bassa100% (1)
- Mass Transfer PartDocument11 pagesMass Transfer Partoctoviancletus100% (1)
- Multicomponent Distillation CalculationsDocument5 pagesMulticomponent Distillation CalculationsPatricia DavidNo ratings yet
- CHE1002 - Process Calculations Material BalanceDocument14 pagesCHE1002 - Process Calculations Material Balanceermias100% (1)
- Mass Transfer PartDocument46 pagesMass Transfer Partoctoviancletus100% (1)
- Drying: CHE133 Heat and Mass Transfer ApplicationsDocument22 pagesDrying: CHE133 Heat and Mass Transfer ApplicationsRA MemijeNo ratings yet
- Mass Transfer PartDocument37 pagesMass Transfer Partoctoviancletus63% (8)
- Gas Absorption: Determining Drag and Flooding FlowsDocument5 pagesGas Absorption: Determining Drag and Flooding FlowsDean Joyce AlborotoNo ratings yet
- Chapter 2 Drying ProcessDocument43 pagesChapter 2 Drying ProcessSyakirin SpearsNo ratings yet
- Energy Savings at DCL PDFDocument83 pagesEnergy Savings at DCL PDFnsprasad88100% (1)
- ALOHA Final Techdoc and QADocument51 pagesALOHA Final Techdoc and QAAubrey WessonNo ratings yet
- Humidification and DryingDocument36 pagesHumidification and Dryingnivedhitha100% (1)
- Drying Technology - An Overview - For ME5202Document134 pagesDrying Technology - An Overview - For ME5202mahe_sce4702No ratings yet
- Drying: Chemical Engineering SeriesDocument54 pagesDrying: Chemical Engineering Serieskmrosario67% (21)
- Appplied Physics AnesthesiaDocument38 pagesAppplied Physics AnesthesiaBogdan CarabasNo ratings yet
- Moisture Content and Drying Rate CalculationsDocument47 pagesMoisture Content and Drying Rate Calculationshels245100% (1)
- Notes EvaporationDocument5 pagesNotes EvaporationPeter Paul Bucsit100% (2)
- Lesson Plan SubAtomicParticles Science8Document5 pagesLesson Plan SubAtomicParticles Science8Joshua James Gitana100% (1)
- Evaporation 1Document23 pagesEvaporation 1nontando sogaNo ratings yet
- Batch Distillation CalculationsDocument47 pagesBatch Distillation CalculationsUdop Charles100% (1)
- Unit OperationDocument17 pagesUnit OperationMohamed KilanyNo ratings yet
- KPMG CEE Hydro Power OulookDocument136 pagesKPMG CEE Hydro Power OulookVladimir MarinkovicNo ratings yet
- GAS ABSORPTION PRINCIPLESDocument15 pagesGAS ABSORPTION PRINCIPLESPeter Paul BucsitNo ratings yet
- EvaporationDocument36 pagesEvaporationPaul Andrew MadlangbayanNo ratings yet
- Problems in Mass TransferDocument3 pagesProblems in Mass TransferAngelica Joyce BenitoNo ratings yet
- TUTORIAL 5 - Modeling Radiation and Natural Convection - 12 Juni 2014Document50 pagesTUTORIAL 5 - Modeling Radiation and Natural Convection - 12 Juni 2014Nadia HandayaniNo ratings yet
- 2014 4M3 DryingDocument40 pages2014 4M3 DryingMina Samy100% (1)
- Humidification and Dehumidification DEHUMIDIFCATION Is The Process in Which The Moisture or Water Vapor or TheDocument7 pagesHumidification and Dehumidification DEHUMIDIFCATION Is The Process in Which The Moisture or Water Vapor or TheAwi ButuanNo ratings yet
- Drying: BKC3492 Separation Process Dr. Zulkifly Jemaat 2020/2021-IDocument50 pagesDrying: BKC3492 Separation Process Dr. Zulkifly Jemaat 2020/2021-IHudaNo ratings yet
- ThermodynamicsDocument2 pagesThermodynamicsRachita Prakash Saraf0% (1)
- Review Key Concepts of Transport PhenomenaDocument7 pagesReview Key Concepts of Transport PhenomenaAdelaida CruzNo ratings yet
- Experiment No. 7 Measurement of Reaction ConversionDocument8 pagesExperiment No. 7 Measurement of Reaction ConversionHoneylet Recaña TayactacNo ratings yet
- Separation Processes: Drying: Che 4M3Document45 pagesSeparation Processes: Drying: Che 4M3Hussaini HamisuNo ratings yet
- Drying of Solids: 18.0 Instructional ObjectivesDocument48 pagesDrying of Solids: 18.0 Instructional ObjectivesIvan MarmilichNo ratings yet
- Chapter#8 CrystallizationDocument49 pagesChapter#8 Crystallization07216738950% (1)
- Reactor Selection CriteriaDocument2 pagesReactor Selection CriteriaJunaid JohnsonNo ratings yet
- Understanding SedimentationDocument23 pagesUnderstanding SedimentationJohnNo ratings yet
- Lewis Randall Rule ProblemsDocument6 pagesLewis Randall Rule ProblemsAshutosh SharmaNo ratings yet
- Problem SetDocument4 pagesProblem SetR SuyaoNo ratings yet
- Computer ApplicationsDocument8 pagesComputer Applicationsapi-3728602100% (1)
- Mass Transfer - II: Distillation Processes ExplainedDocument39 pagesMass Transfer - II: Distillation Processes ExplainedSMIT CHRISTIANNo ratings yet
- 9 EvaporationDocument82 pages9 Evaporationsanjay YadavNo ratings yet
- Mass Transfer Operations 1: List of Books Information of CrystallizationDocument13 pagesMass Transfer Operations 1: List of Books Information of CrystallizationZaid MansuriNo ratings yet
- Liquid-Liquid Extraction - 17 Sept 2020 - 4 PDFDocument32 pagesLiquid-Liquid Extraction - 17 Sept 2020 - 4 PDFshubham100% (1)
- SedimentationDocument9 pagesSedimentationAutumn JohnsonNo ratings yet
- Drying PSDocument10 pagesDrying PSVan Vesper DulliyaoNo ratings yet
- D D D D DDocument5 pagesD D D D Drazzee yuchengkoNo ratings yet
- Experiment: Packed Distillation ColumnDocument4 pagesExperiment: Packed Distillation Columnnhalieza1067No ratings yet
- Review CheDocument4 pagesReview CheSheena GagarinNo ratings yet
- Heat TransferDocument71 pagesHeat TransferZeus OlympusNo ratings yet
- Practice Problems in ADSORPTION and ION EXCHANGE - SolutionsDocument8 pagesPractice Problems in ADSORPTION and ION EXCHANGE - SolutionsJenna Brasz100% (2)
- ChE 126 SEPARATION PROCESSES: ADSORPTION ISOTHERMS (39Document27 pagesChE 126 SEPARATION PROCESSES: ADSORPTION ISOTHERMS (39Anthony Justin EdmaNo ratings yet
- H81SPF Leaching and Washing FundamentalsDocument14 pagesH81SPF Leaching and Washing Fundamentalsimmo50% (2)
- Tutorial 1Document2 pagesTutorial 1Syakirin Spears0% (2)
- Un1 MergedDocument467 pagesUn1 MergedHariHaran KNo ratings yet
- AssigDocument2 pagesAssigZakwan0% (1)
- Psychrometrics Drying Problems SEODocument5 pagesPsychrometrics Drying Problems SEOStephanie Torrecampo Delima100% (2)
- 3 - Vegetable Oil and BiofuelsDocument15 pages3 - Vegetable Oil and BiofuelsVenus Abigail GutierrezNo ratings yet
- Tray Drying Experiment: Effects of Air Velocity on Drying RateDocument13 pagesTray Drying Experiment: Effects of Air Velocity on Drying RateSrinyanavel ஸ்ரீஞானவேல்75% (4)
- Mass Transfer Ecp 224: Unit 4: LeachingDocument53 pagesMass Transfer Ecp 224: Unit 4: LeachingTapiwa KapondaNo ratings yet
- Heat Transfer Lab Manual 2015-16Document99 pagesHeat Transfer Lab Manual 2015-16Harshit Sinha100% (1)
- HumidificationDocument5 pagesHumidificationAlbert Junior EvangelistaNo ratings yet
- Double Pipe Heat Exchanger: Experiment 9Document6 pagesDouble Pipe Heat Exchanger: Experiment 9Jelain HumarangNo ratings yet
- Topic 0 DryingDocument43 pagesTopic 0 DryingJA NableNo ratings yet
- DRY Chapter PDFDocument22 pagesDRY Chapter PDFrameshpavalar123No ratings yet
- Analytical Chemistry GuideDocument120 pagesAnalytical Chemistry GuideJomed BarallasNo ratings yet
- Notes DiffusionDocument60 pagesNotes DiffusionPeter Paul BucsitNo ratings yet
- Engineering Properties of Soil and Rock ChapterDocument38 pagesEngineering Properties of Soil and Rock ChapterjonaspdNo ratings yet
- Cyclone Separator CFDDocument5 pagesCyclone Separator CFDAMARESH BADIGERNo ratings yet
- PHYSICS 149: Lecture 5: - Chapter 2Document28 pagesPHYSICS 149: Lecture 5: - Chapter 2Sourav PandaNo ratings yet
- Ancient Method-Black GoldDocument2 pagesAncient Method-Black GoldJoelLadjoNo ratings yet
- 5CE4-05 WRE Guess Paper PDFDocument4 pages5CE4-05 WRE Guess Paper PDFYogesh PrajapatiNo ratings yet
- Plastic Marine PollutionDocument3 pagesPlastic Marine PollutionDivine Grace CabungcagNo ratings yet
- Summative TEstDocument14 pagesSummative TEstxoxkakidoxoxNo ratings yet
- Pyrogen System - Referance ManualDocument47 pagesPyrogen System - Referance ManualSuresh GujjarNo ratings yet
- 2014 Environmental Impacts Caused by Phosphate Mining and Ecological Restoration A Case History in Kunming, China.Document16 pages2014 Environmental Impacts Caused by Phosphate Mining and Ecological Restoration A Case History in Kunming, China.Fábio Paiva da SilvaNo ratings yet
- BTD-Final Lesson PlanDocument22 pagesBTD-Final Lesson PlanSunil BajantriNo ratings yet
- Ground Improvement PPT ISquareRDocument19 pagesGround Improvement PPT ISquareRsamNo ratings yet
- Solar Energy System for CoachesDocument46 pagesSolar Energy System for CoachesVenkatesh VenkateshNo ratings yet
- Lecture 345Document5 pagesLecture 345Thyrone Jay D RamirezNo ratings yet
- Explore Samal Island's Powdery Beaches and Marine ReefsDocument5 pagesExplore Samal Island's Powdery Beaches and Marine ReefsStephany JulaoNo ratings yet
- 1PHYSICS I - FORCES AND EQUILIBRIUM - STUDENTS - 2020 (3) (Autosaved)Document16 pages1PHYSICS I - FORCES AND EQUILIBRIUM - STUDENTS - 2020 (3) (Autosaved)Aser SerNo ratings yet
- Oau Post Utme Past Question and AnswerDocument23 pagesOau Post Utme Past Question and AnswerFoluso Adeosun100% (1)
- 18 - 70 Heppy Yessya PutriDocument6 pages18 - 70 Heppy Yessya PutriHeppy YessyaNo ratings yet
- Intro To BiomesDocument5 pagesIntro To Biomesapi-235669157No ratings yet
- 1 1 AP Homework Answer Key-1Document3 pages1 1 AP Homework Answer Key-1Kaitlyn CabreraNo ratings yet
- SDS US - UVEX Fog Eliminator ClothDocument6 pagesSDS US - UVEX Fog Eliminator ClothRaul RodriguezNo ratings yet
- Factors Causing Slope FailuresDocument2 pagesFactors Causing Slope FailuresAqir SyamilNo ratings yet
- Question and Answers OscillationDocument22 pagesQuestion and Answers OscillationVishal BhanawatNo ratings yet