Professional Documents
Culture Documents
Discussion Exp 5
Uploaded by
Abdul AddaharyCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Discussion Exp 5
Uploaded by
Abdul AddaharyCopyright:
Available Formats
Result & Discussion
Part A
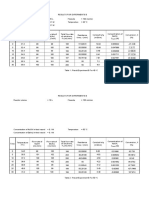
Metal as reducing agents
Test tube no
1 (copper)
0.1 M CuSO4
No reaction
2 (magnesium)
Brown deposited
3 (Zinc)
Corroded
4 (Steel wool)
Rusting
3 M H2SO4
The color of
copper fading
Dissolves and
produce pop sound
Corroded and
produce pop sound
Grey precipitate
deposited
0.1 M ZnSO4
No reaction
Zinc metal
deposited
No reaction
No reaction
Metal as reducing agents
CuSO4 (aq) + Cu (s)
CuSO4 (aq) + Mg (s)
CuSO4 (aq) + Zn (s)
CuSO4 (aq) + Fe (s)
CuSO4 (aq) + Cu (s)
MgSO4 (aq) + Cu (s)
MgSO4 (aq) + Cu (s)
FeSO4 (aq) + Cu (s)
H2SO4 (aq) + Cu (s)
H2SO4 (aq) + Mg (s)
H2SO4 (aq) + Zn (s)
H2SO4 (aq) + Fe (s)
H2SO4 + Cu
MgSO4 + H2
ZnSO4 +H2
FeSO4 + H2
ZnSO4 (aq) + Cu (s)
ZnSO4 (aq) + Cu (s)
ZnSO4 (aq) + Mg (s)
MgSO4 + Zn
ZnSO4 (aq) + Zn (s)
ZnSO4 (aq) + Zn (s)
ZnSO4 (aq) + Fe (s)
ZnSO4 + Fe (s)
Arrangement of the four metals and hydrogen in the order of their activities beginning
with the best reducing agent and ending with the poorest reducing agent.
1. Mg
2. Zn
3. Fe
4. Cu
5. H
PART B
Solution for redox reaction.
Test tube
\1
Solution X
0.1 M KMnO4
+
2 drops 6 MH2SO4
Observation and redox reaction equation
0.1 M Fe (NH4)2(SO4)
0.1 M K2C2O4
Purple to reddish brown
0.1 M KMnO4
+
2drops 6 M H2SO4
0.1 M K2Cr2O7
+
From purple to
colourless
Yellow participated
2 drops 6 MH2SO4
0.1 M K2Cr2O7
+
Yellow to light
2 drops 6 MH2SO4
yellow
The experiment used to observe and predict products of oxidation-reactions. Besides that,
its also used to determine the relative reactivity of a series of metallic elements. Redox
reaction is about transfer of electrons, the lower the position of the element inside the
electrochemical series (ECS) the lower electro positivity. Redox is a combination of two
words, reducing agent and oxidizing agent. Reducing agent donates electrons for the
reduction of another substance. An oxidizing agent is a substance that gains electrons and
causes the oxidation of another substance in a redox reaction.
Oxidation reaction involves an increase in oxidation number, while reduction reaction
involves a decrease in oxidation number. These redox reactions always occur in pair. When
something get oxidized, another agent gains those electron, acting as the oxidizing agent, and
get reduced in process. When a substance gets reduced, it gains electron from the other agent,
acting as reducing agent, which in the process get oxidized. Furthermore, redox reaction also
can be determined by the changing of the colour of the solution. When redox reaction is occur
the colour of the solution will change.
Based on the result, redox reaction do not occur at the metal that same with the solution,
as example, when Copper sulphate reacts with solid copper there is no reaction occur. But the
result is difference when the square metal been tested with sulphuric acid, as example, when
magnesium and zinc react with sulphuric acid, a pop sound is produced. When the zinc
sulphate being used with square metal to test the redox reaction, only magnesium corroded.
Based on the result, the objectives are achieved. This is because the redox reactions are
occur during the experiment. Redox reaction is about the reducing agent and the oxidising
agent, as example, when react the sulphuric acid with the magnesium, the magnesium
replaced the position of the hydrogen ion. This is because the higher the position of the metal
inside the electrochemical series (ECS) the more electro positive the metal.
When the experiment is being conducted, there are some error occur. Some of the
error is the amount of the sulphuric acid need to be dropped into the test tube has excess
because it is being done with carelessly. Next, the observation of the result has been done
with carelessly until the result is different with the actual result. Lastly, the test tube doesnt
being clean up properly until its affect the result of the experiment.
You might also like
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (890)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- 2C-B Synthesis Without LAH PDFDocument4 pages2C-B Synthesis Without LAH PDFatomosco100% (3)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- Rules of ThumbDocument105 pagesRules of ThumbEllen DawitriNo ratings yet
- On CNG-20-12-19Document31 pagesOn CNG-20-12-19laxmikant100% (1)
- Hydro ProcessingDocument56 pagesHydro ProcessinggeorgiadisgNo ratings yet
- Cooling Water Treatment Chlorination WorkoutDocument3 pagesCooling Water Treatment Chlorination WorkoutMohsin ModiNo ratings yet
- Compressed Air Filters LeafletDocument20 pagesCompressed Air Filters LeafletAnonymous FZs3yBHh7No ratings yet
- SUMMARY REF Exp1Document2 pagesSUMMARY REF Exp1Abdul AddaharyNo ratings yet
- WBB10202 Innovation Management Lecturer: Suhaiza Ngah Topic 1: Perspectives On InnovationDocument16 pagesWBB10202 Innovation Management Lecturer: Suhaiza Ngah Topic 1: Perspectives On InnovationAbdul AddaharyNo ratings yet
- Tutorial 1: Introduction To MarketingDocument2 pagesTutorial 1: Introduction To MarketingAbdul AddaharyNo ratings yet
- FORM 2 - Proposal Assessment - FYP 1Document4 pagesFORM 2 - Proposal Assessment - FYP 1Abdul AddaharyNo ratings yet
- OB DominosDocument3 pagesOB DominosAbdul AddaharyNo ratings yet
- Introduction and MethodDocument6 pagesIntroduction and MethodAbdul AddaharyNo ratings yet
- Economic of REDocument3 pagesEconomic of REAbdul AddaharyNo ratings yet
- Intellectual Property (PAPER SEMINAR)Document10 pagesIntellectual Property (PAPER SEMINAR)Abdul AddaharyNo ratings yet
- Wave PowerDocument18 pagesWave PowerAbdul AddaharyNo ratings yet
- Tutorial 1: Introduction To MarketingDocument2 pagesTutorial 1: Introduction To MarketingAbdul AddaharyNo ratings yet
- Tutorial 1 PC July 2018Document5 pagesTutorial 1 PC July 2018Abdul AddaharyNo ratings yet
- Tutorial 2-Part 1Document2 pagesTutorial 2-Part 1Abdul AddaharyNo ratings yet
- Economic of REDocument3 pagesEconomic of REAbdul AddaharyNo ratings yet
- Literature RiviewDocument3 pagesLiterature RiviewAbdul AddaharyNo ratings yet
- Peer Evaluation FormDocument3 pagesPeer Evaluation FormAbdul AddaharyNo ratings yet
- Checklist PMDocument4 pagesChecklist PMAbdul AddaharyNo ratings yet
- Resultndiscussion 151126101738 Lva1 App6891Document2 pagesResultndiscussion 151126101738 Lva1 App6891Abdul AddaharyNo ratings yet
- CalcII ArcLength PracticeDocument2 pagesCalcII ArcLength PracticeshulandNo ratings yet
- Overview of Solid Processing Technologies for Palm Oil BiomassDocument3 pagesOverview of Solid Processing Technologies for Palm Oil BiomassAbdul AddaharyNo ratings yet
- Checklist PMDocument3 pagesChecklist PMAbdul AddaharyNo ratings yet
- Lab Report Cover Page 2017Document1 pageLab Report Cover Page 2017Abdul AddaharyNo ratings yet
- Conclusions PilotplantDocument4 pagesConclusions PilotplantAbdul AddaharyNo ratings yet
- Anticline ImpermeableDocument4 pagesAnticline ImpermeableAbdul AddaharyNo ratings yet
- Theory: P P or P PDocument4 pagesTheory: P P or P PAbdul AddaharyNo ratings yet
- Results For Experiments 2bDocument13 pagesResults For Experiments 2bAbdul AddaharyNo ratings yet
- Determination of Diesel Flash Point Experiment ResultsDocument3 pagesDetermination of Diesel Flash Point Experiment ResultsAbdul AddaharyNo ratings yet
- Pilot Plant Assignment 1Document3 pagesPilot Plant Assignment 1Abdul AddaharyNo ratings yet
- Refrence and ConclusionDocument2 pagesRefrence and ConclusionAbdul AddaharyNo ratings yet
- RC and RLC Series Circuit ImpedanceDocument2 pagesRC and RLC Series Circuit ImpedanceAbdul AddaharyNo ratings yet
- Hydrate Formation Conditions of Sour Natural GasesDocument3 pagesHydrate Formation Conditions of Sour Natural Gasesanon_936836736No ratings yet
- Shell Dep Offshore Chloride Stress Corrosion - Google SearchDocument2 pagesShell Dep Offshore Chloride Stress Corrosion - Google SearchIvan GutierrezNo ratings yet
- Membrane Reactor TechnologyDocument140 pagesMembrane Reactor TechnologyMohamed Laíd SakhriNo ratings yet
- Operating Problems With Trays SolvedDocument57 pagesOperating Problems With Trays Solvedsadaf munirNo ratings yet
- Fuel PropertiesDocument17 pagesFuel PropertiesJasjit SinghNo ratings yet
- lw450 Service ManualDocument36 pageslw450 Service ManualAngelNo ratings yet
- Liquid FuelsDocument12 pagesLiquid FuelsCharles MayoNo ratings yet
- Edurev: Solved Problems - Chemical Kinetics, Class 12, ChemistryDocument1 pageEdurev: Solved Problems - Chemical Kinetics, Class 12, ChemistryNicole Ann KimmayongNo ratings yet
- Gujarat Technological UniversityDocument2 pagesGujarat Technological Universityfeyayel988No ratings yet
- Palatal' In9re Iierrs: United States Patent (191Document12 pagesPalatal' In9re Iierrs: United States Patent (191Anonymous VJFlyRY0No ratings yet
- Chemical Process Principles Assignment 2 SolutionsDocument2 pagesChemical Process Principles Assignment 2 SolutionsHafizuddin AdzharNo ratings yet
- Refrigeration & Heat PumpDocument36 pagesRefrigeration & Heat PumpMd. Ahsanur RahmanNo ratings yet
- MCQ Astm Distillation: Abdul Majeed Ahmed Hossam IsmailDocument4 pagesMCQ Astm Distillation: Abdul Majeed Ahmed Hossam IsmailAbdul Majeed AhmedNo ratings yet
- PC Question Paper Nov 2021Document4 pagesPC Question Paper Nov 2021venkatesan sivaramuNo ratings yet
- 13 TaylorDocument12 pages13 TaylorAlberto SanchezNo ratings yet
- CH E 511A: Separation Processes and Introduction To Particulate Technology LeachingDocument8 pagesCH E 511A: Separation Processes and Introduction To Particulate Technology LeachingKhayie VictorianoNo ratings yet
- Lecture 1Document8 pagesLecture 1Yogesh PatilNo ratings yet
- To Add or Subtract Values, Dimensional Quantities Must Be The Same. Since T Is in K, 4 Must Also Be in KDocument3 pagesTo Add or Subtract Values, Dimensional Quantities Must Be The Same. Since T Is in K, 4 Must Also Be in KdddddNo ratings yet
- IEC/ATEX Electrical Equipment for Hazardous EnvironmentsDocument1 pageIEC/ATEX Electrical Equipment for Hazardous EnvironmentsVassoula Dar100% (1)
- Critical Review of Catalysis For Ethylene OxychlorinationDocument22 pagesCritical Review of Catalysis For Ethylene OxychlorinationHồ Thị LànhNo ratings yet
- SPR Review CO2 CH4Document62 pagesSPR Review CO2 CH4erwin_carryNo ratings yet
- Carbon Dioxide (CO2) Applications and Uses:: 2-Metals IndustryDocument15 pagesCarbon Dioxide (CO2) Applications and Uses:: 2-Metals Industryahmed atwaNo ratings yet
- Introduction To Chemie-Tech - A21Document35 pagesIntroduction To Chemie-Tech - A21Sameer BawaNo ratings yet