Professional Documents
Culture Documents
E45 Laboratory4
Uploaded by
nickOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
E45 Laboratory4
Uploaded by
nickCopyright:
Available Formats
E 45 Laboratory Manual
Laboratory 4
Heat Treatment of Steel
Objectives
1. To understand the effect of thermal processing (heat treatment) on both the microstructure and properties (hardness) of steel
2. To understand the application of time-temperature-transformation (TTT) curves in ferrous metallurgy
!
Overview
T (C)
1600
tural components in the construction and transporta-
1538
1495
1400 1394
1300
tion industries to specialty tools in the medical indus-
1200
try. Its properties vary widely, depending upon the
1100
thermomechanical processing cycles to which steel
1000
components are subjected, and their exposure to in-
900
Steel is one of the most versatile engineering materials, used in a wide range of applications from struc-
service conditions. This lab offers a survey of the
role of heat treatment in the development of microstructures and properties to optimize engineering
performance in ferrous alloys.
!
!
1500
L
1227
1148
2.11
6.67
4.30
910
800
727
700 0.02 0.77
600
6.67
Cementite
500
400
300
200
Figure 1 The Fe-C phase diagram, to 6.67wt.%
C, from Metals Handbook, 8th edition, Volume 8,
ASM, Metals Park, Ohio, (1973).
Copyright 2014, Berkeley
100
0
Fe
3
4
wt % C
Fe3C
Professor Lane W. Martin
Laboratory 4: Heat Treatment of Steel
Equipment
Heat Treatment
1. ASTM 1045 (0.45 wt.% C) cold-rolled steel specimen
2. Furnace and wire for suspending specimen in hot zone
3. Thermocouple [chromel + alumel]
4. Computer data acquisition system
Metallography
5. Polishing clamp, wet belt grinder , and polishing papers (240, 320, 400, 600)
6. Electropolisher, with polishing solution consisting of 60 ml distilled water + 50 ml butyl cellulose + 350 ml ethyl
alcohol, 95%, (and just before using, add)+ 40 ml perchloric acid, 60%
7. Glass slide, specimen press
8. Optical microscope
Hardness Testing
9. Rockwell hardness tester
10. Measurement ruler
Background
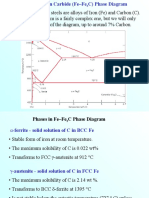
Steel is an alloy of iron (Fe) and carbon (C), with concentrations of carbon from 0.1 to 2.0 weight percent. Carbon is
soluble in Fe because the C atoms are small enough to fit into interstitial locations between Fe atoms without too
much distortion of the lattice. Carbon is soluble in the FCC phase of Fe (called austenite or gamma-Fe, or ) up to
approximately 2%. However, in the BCC phase of Fe (called ferrite or alpha-Fe or ), the maximum solubility is only
about 0.02%. These phases are shown on the equilibrium phase diagram in Fig. 1.
When austenite is cooled below a critical temperature called the eutectoid temperature (727C), it becomes unstable.
Most of the Fe tends to precipitate as nearly pure ferrite and most of the C tends to aggregate in the intermetallic compound Fe3C (cementite). The transformation of austenite requires redistribution of C atoms from a random solid solution to one in which nearly all C is contained in the Fe3C precipitates.
At temperatures just below the eutectoid decomposition temperature the driving force for the transformation is low,
and nucleation of the two new phases, ferrite and cementite, is slow. It is therefore necessary to hold the specimen at
temperature for a considerable time to allow the transformation to take place in those regions of the specimen that are
just below 727C. The lower the temperature, the greater the driving force for the reaction to occur, causing a higher
nucleation rate. At temperatures below about 540C, however, the rate of transformation again becomes slower. This
page 2
" of 7
"
Laboratory 4: Heat Treatment of Steel
is due to the decreasing mobility of C atoms in the austenite. In order for the transformation to continue, C atoms
must diffuse away from the growing ferrite regions to the growing Fe3C regions.
If the austenite is cooled so rapidly that there is not enough time for nucleation and growth of ferrite and Fe3C, an entirely different kind of transformation takes place called the "martensitic" transformation. In this reaction the FCC
austenite changes instantly by a shear mechanism to a body-centered tetragonal structure, trapping C in the new
phase, called martensite.
These reactions are best illustrated in a figure known as a time-temperature-transformation (TTT) diagram. It portrays
the decomposition kinetics of austenite, obtained by isothermally holding a sample for different times until the reaction is complete. An example is shown in Fig. 2.
800 804
727
700
500
e 3C
400
300
200
+ Fe3C
+F
Temperature (C)
600
Ms
Mf
100
0
0.1
1 sec.
1
1 min.
10
102
Time (seconds)
1 hour
103
1 day
104
105
Figure 2 Schematic TTT curve for a hypoeutectoid plain carbon steel. The pro-eutectoid ferrite constituent
forms below 804C, and the eutectoid constituent below 727C. Both the start (Ms) and the finish (Mf) of the
athermal martensitic transformation are seen at lower temperatures. From The Making, Shaping, and Treating of Steel, H.E. McGannon (Ed.), 9th Edition, United States Steel Corporation, Pittsburgh, (1971), p. 1087.
The mechanical properties of steel depend upon the dispersion of C atoms or of cementite particles in the Fe matrix.
Since redistribution of C atoms requires diffusion and since the rate of diffusion decreases exponentially with decreasing temperature, the coarsest dispersions of Fe3C and of ferrite result from the transformation that is allowed to take
place at high temperatures, just below the critical temperature. The resulting eutectoid product is known as coarse
pearlite. If the transformation is forced to take place at lower and lower temperatures by increasing the rate of cooling, then finer and finer dispersions of the hard brittle carbon-rich phase (Fe3C) and the relatively soft ductile ferrite
are produced, known as fine pearlite. If the transformation takes place at temperatures below about 400C, the ferrite
and cementite form as extremely fine needles. This microstructure is known by another name, "bainite."
page 3
" of 7
"
Laboratory 4: Heat Treatment of Steel
The hardness of steel generally increases with the fineness of the dispersion of the phases in the microstructure. If the
rate of cooling is increased still further, the precipitation of carbon as Fe3C can be suppressed altogether, forming
martensite, in which the C atoms are still distributed nearly at random. This is the hardest structure possible for any
given steel. However, since martensite has low ductility, many machine parts are first rapidly cooled to produce
martensite, then tempered by holding at slightly elevated temperatures to redistribute the carbon as fine precipitates.
The resulting microstructure is known as tempered martensite and benefits from some restored ductility.
Experimental Procedures
Part I Heat Treatment
Each group will be given a sample of 1045 cold rolled steel.
The sample will first be heated uniformly in the
austenitic region of the phase diagram (Fig. 1). The sample will then be heated under conditions resulting in a temperature gradient along its length, as illustrated in Fig. 3.
!
!
!
Thermocouple
Top view:
sample
aligned
with
furnace
slot
Figure 3 Schematic experimental setup.
The thermocouple tip rests in a small depression in the top of the sample, which is suspended by a Nichrome wire. Inset at left
shows crucial alignment of sample with the
rectangular slot at the bottom of the furnace.
In center frame, the entire sample is heated
to 1050C and held for 5 minutes. At right,
the sample is lowered into the water, submerged 1/8 inch, to establish temperature
gradient (900C at top) and held for 15 minutes. It may be necessary to replenish the
water supply due to evaporative losses.
1/8 inch
gap to
water
and
under
water
!
!
!
!
page 4
" of 7
"
Laboratory 4: Heat Treatment of Steel
1. Suspend both the steel sample on its Nichrome wire tether and the thermocouple using an adjustable lab clamp
as shown in Fig. 3. Note carefully the relative orientation of the sample and the rectangular slot through the furnace. Adjust so there is adequate clearance for the sample to pass smoothly through the furnace slot by simple
vertical motion of the lab clamp. This will be critical during the later stages of this experiment.
2. Position the sample in the center of the furnace heating coils.
3. Connect the thermocouple to the data acquisition interface box.
4. Turn on power to the furnace and start the data acquisition program to begin recording temperature data.
5. Heat to 1050C and hold at this temperature for at least 5 minutes.
6. Quickly drop the sample so that it is contacts the water with approximately " submerged, as shown in Fig. 3.
7. Adjust the power input so that the temperature at the top of the sample is 900C. If the water level decreases,
carefully add more water with the squirt bottle, avoiding any splashing of water into the furnace. Hold the temperature at 900C for 15 minutes by adjusting the power source as needed.
8. After 15 minutes, quench the sample by dropping it quickly into the water and vigorously agitating it until cool.
9. Turn off the power. When the furnace has cooled to about 50C, remove the sample and terminate data recording.
Part 2 Metallographic Polishing
Mount the sample in the holding clamp and remove the oxide scale by pressing the sample lightly on the grinder to
obtain a flat surface on one side. Do this for both sides of the sample. Then grind by hand, moving the sample in a
straight line (not a circular motion) on successively finer paper down to 600 grit. For each change of grit paper,
change the orientation of the sample by 90 and continue sanding until the orthogonal scratch marks disappear. Remember to wash the sample between paper changes to remove all remnant grit.
Part 3 Metallographic Etching
Samples must be electropolished to a specular finish as the final step in metallographic preparation, which also provides a light etch to delineate grain boundaries, dislocations that intersect the surface, and interphase boundaries. Afterwards, quickly wash the sample in running water, rinse with alcohol and dry under the hot air blower. Be careful
not to touch the polished surface at any time, or else the fine grinding and polishing steps will have to be repeated.
Part 4 Hardness Indentation Test
Take a series of Rockwell A hardness measurements (60 kg major load) at -inch intervals along the long dimension of the sample, to probe the effect of the temperature gradient on the mechanical properties of the sample. Record
the results, approximately 16 hardness values total, on the data sheet.
page 5
" of 7
"
Laboratory 4: Heat Treatment of Steel
Part 5 Metallography
Mount the sample on a glass slide with plasticene using the specimen press to level the surface. This will keep the
sample in focus as it is moved laterally with the microscope stage. Choose five (5) areas along the edges where the
hardness readings are different and/or where the microstructure looks different. Sketch the microstructures at each of
the five areas. Notice the difference in grain sizes, the density of defects (dislocations), and the morphology of the
microconstituents. Sketch these and record your observations in your lab notebook to support your sketches. Also,
look for the answers to the questions in the Lab Report section when examining the sample.
DataSheet 1 Hardness Measurements
Position
Major Load
Minor Load
Hardness
1
2
16
This datasheet should be set up in a spreadsheet application to facilitate the use of a graphing function as called for in
the Questions below.
!
!
References
1. G. Krauss, Principles of Heat Treatment of Steel, American Society for Metals, Metals Park, OH, (1980).
2. W.T. Lankford, et al., Eds., The Making, Shaping, and Treating of Steel, 10th edition, Association of Iron and Steel
Engineers, Pittsburgh, PA, (1985).
3. Metals Handbook, 9th edition, Vol. 4, Heat Treating, American Society for Metals, Metals Park, OH, (1981).
page 6
" of 7
"
Laboratory 4: Heat Treatment of Steel
Lab Report
Precise data presentation is expected, accompanied by relevant references. Answers to the following questions are
also required.
Question 1
For each of the five (5) different microstructures evident in your results, draw a representative cooling curve on a TTT
diagram corresponding to the thermal history associated with the specific area of the sample where the microstructure
was observed. Use a separate TTT diagram for each of the five cooling curves, but show them all at the same scale.
Verify the phases present in each microstructure and compare to your cooling curves. Specify the order in which the
phases appeared, and refer to the phase diagram as necessary to validate your conclusions.
Question 2
Make a plot of hardness versus distance from the 900C end of the sample. Use your hardness data to rank martensite, ferrite, and pearlite from the hardest to the softest. Support your results by discussing the nature of the microstructure, crystal structure, and chemical bonding associated with all three phases (martensite, ferrite, and carbide).
Question 3
Does the transformation of austenite into ferrite begin at the austenite grain boundaries and propagate inward, or does
it begin inside the grains and propagate outward toward the boundaries? Include a sketch of the microstructure from
the appropriate part of the specimen illustrating the answer to this question. Explain.
Question 4
How could a structure containing only ferrite and martensite be produced in a 1045 steel specimen? Illustrate on a
TTT diagram.
Question 5
Could you heat treat a 1045 steel specimen so that it contains only ferrite? Describe how this can be achieved, or explain why it cannot be done.
Question 6
Why isn't martensite on the Fe-Fe3C phase diagram of Fig. 1? Why doesn't its absence from the phase diagram prevent it from being an important engineering material?
page 7
" of 7
"
You might also like
- Tap Yourself FreeDocument134 pagesTap Yourself Freenguyenhavn100% (2)
- Heat Treatment by Quenching - DiagramsDocument20 pagesHeat Treatment by Quenching - Diagramssunilmathew4477No ratings yet
- Allen Bradley Power Monitor 3000 Manual PDFDocument356 pagesAllen Bradley Power Monitor 3000 Manual PDFAndrewcaesar100% (1)
- Heat Treatment Definition and ObjectivesDocument56 pagesHeat Treatment Definition and ObjectivesAakarsh RastogiNo ratings yet
- TTT DiagramDocument6 pagesTTT DiagramDeepa PujariNo ratings yet
- TTT Phase DiagramDocument9 pagesTTT Phase Diagramhari krishnaNo ratings yet
- Physical Metallurgy-18 Heat Treatment of SteelDocument7 pagesPhysical Metallurgy-18 Heat Treatment of SteelDSGNo ratings yet
- Report Heat Treatment Eng Lab 3Document7 pagesReport Heat Treatment Eng Lab 3khalifawhan43% (7)
- Principles of Heat Treating of SteelsDocument30 pagesPrinciples of Heat Treating of Steelssatish_trivediNo ratings yet
- Induction Hardening - Interpretation of Drawing & Testing PDFDocument4 pagesInduction Hardening - Interpretation of Drawing & Testing PDFrajesh DESHMUKHNo ratings yet
- Iron-carbon phase diagram guideDocument9 pagesIron-carbon phase diagram guideNagamuthu PandianNo ratings yet
- Heat Treatment TTT DiagramsDocument3 pagesHeat Treatment TTT Diagramsferrumdg100% (1)
- The Working of Steel: Annealing, Heat Treating and Hardening of Carbon and Alloy SteelFrom EverandThe Working of Steel: Annealing, Heat Treating and Hardening of Carbon and Alloy SteelNo ratings yet
- Closed-Book Practice-Ch 10 (2017!08!08)Document12 pagesClosed-Book Practice-Ch 10 (2017!08!08)Juan0% (1)
- Time Temperature Transformation (TTT) Diagrams PDFDocument108 pagesTime Temperature Transformation (TTT) Diagrams PDFSerkan Apay100% (1)
- The Dedication of the Broken Hearted SailorDocument492 pagesThe Dedication of the Broken Hearted SailorGabriele TorresNo ratings yet
- Phase Transformation in Metals: Dr. Aneela WakeelDocument29 pagesPhase Transformation in Metals: Dr. Aneela WakeelmazharNo ratings yet
- E45 Lab 6 Heat Treatment of SteelDocument8 pagesE45 Lab 6 Heat Treatment of SteelAlisha PowerNo ratings yet
- 8.heat TreatmentDocument7 pages8.heat Treatmentrohan_n_desai100% (2)
- Thermal Lab 1Document6 pagesThermal Lab 1Muhammad ZulhilmiNo ratings yet
- Fe-Fe3C Phase Diagram GuideDocument55 pagesFe-Fe3C Phase Diagram GuideMrDOTNo ratings yet
- BG2802 Heat Treatment and Mechanical Properties of SteelsDocument11 pagesBG2802 Heat Treatment and Mechanical Properties of SteelsVenus LimNo ratings yet
- Fe-Fe3C Phase Diagram ExplainedDocument6 pagesFe-Fe3C Phase Diagram Explainedolid_zoneNo ratings yet
- 7-Hardenability of SteelDocument4 pages7-Hardenability of SteelMuhammad ZulhilmiNo ratings yet
- Publication 4 11889 199Document9 pagesPublication 4 11889 199Mulia AridhoNo ratings yet
- Anne A Ling and Normalizing of SteelDocument5 pagesAnne A Ling and Normalizing of SteelTareef HashNo ratings yet
- Time Temperature Transformation With ReferncesDocument12 pagesTime Temperature Transformation With ReferncesEllie BrooklynNo ratings yet
- TEST 1marking Guide: Mass Effect of Heat-Treatment. This Eliminates The More Costly Need To Quench-Harden With ADocument5 pagesTEST 1marking Guide: Mass Effect of Heat-Treatment. This Eliminates The More Costly Need To Quench-Harden With ARhea GaiaNo ratings yet
- Time Temperature Transformation With ReferncesDocument12 pagesTime Temperature Transformation With ReferncesEllie BrooklynNo ratings yet
- Heat Treatment of Heat Treatment of Heat Treatment of Steel Alloys Heat Treatment of Steel AlloysDocument42 pagesHeat Treatment of Heat Treatment of Heat Treatment of Steel Alloys Heat Treatment of Steel AlloysengrumairshahidNo ratings yet
- Engineering Metallurgy Chapter-8 Ref: Introduction To Physical MetallurgyDocument34 pagesEngineering Metallurgy Chapter-8 Ref: Introduction To Physical MetallurgyMD Al-AminNo ratings yet
- TTT DiagramDocument1 pageTTT DiagramaasattiNo ratings yet
- Iron Carbon Phase DiagramDocument4 pagesIron Carbon Phase DiagramMizanur RahmanNo ratings yet
- Heat Treatment Effects on Low Carbon SteelDocument9 pagesHeat Treatment Effects on Low Carbon SteelAhmad Farhan F1120No ratings yet
- Ch-8 Compatibility ModeDocument58 pagesCh-8 Compatibility Modedreamgurl9011No ratings yet
- Capili Jefferson 11Document16 pagesCapili Jefferson 11Christian Al EncarnacionNo ratings yet
- 05 Heat Treatments To Produce Ferrite and PerliteDocument23 pages05 Heat Treatments To Produce Ferrite and PerliteRicardo Fidel Duarte SánchezNo ratings yet
- EMAT 10 (2k21)Document41 pagesEMAT 10 (2k21)Kumail AbbasNo ratings yet
- E45 Lab5Document16 pagesE45 Lab5Kristine TjungNo ratings yet
- Iron Carbide Phase Diagram AnalysisDocument35 pagesIron Carbide Phase Diagram AnalysisbotobotoakbarNo ratings yet
- Chap 6 TTT Diagram (New)Document26 pagesChap 6 TTT Diagram (New)eeit_nizamNo ratings yet
- Formation of Delta Ferrite in 9 WT.% CR Steel Investigated by In-Situ X-Ray Diffraction Using Synchrotron RadiationDocument9 pagesFormation of Delta Ferrite in 9 WT.% CR Steel Investigated by In-Situ X-Ray Diffraction Using Synchrotron Radiationsmallik3No ratings yet
- TempleDocument17 pagesTemplesan moedanoNo ratings yet
- Applying Computer Simulation in Improving Heat Treating Condition of Thin High-Carbon Steel PartsDocument5 pagesApplying Computer Simulation in Improving Heat Treating Condition of Thin High-Carbon Steel PartssathishelakkiyaNo ratings yet
- Homework 14 Solutions Spring 2001Document2 pagesHomework 14 Solutions Spring 2001Ikhwan Wf Miscellaneous AveroesNo ratings yet
- Heat Treatment GuideDocument24 pagesHeat Treatment GuidemiteshNo ratings yet
- Materi Kuliah Heat TreatmentDocument16 pagesMateri Kuliah Heat TreatmentGama Kus RohkmatullohNo ratings yet
- High-temperature tensile properties and thermal cracking of ferritic spheroidal graphite cast ironDocument9 pagesHigh-temperature tensile properties and thermal cracking of ferritic spheroidal graphite cast ironRegiane SenaNo ratings yet
- Iron Carbon DiagramDocument8 pagesIron Carbon Diagramashok pradhanNo ratings yet
- 1 - Heat TreatmentDocument61 pages1 - Heat TreatmentMohamed Karim MohamedNo ratings yet
- Phase Transformation in AISI 410 Stainless Steel PDFDocument10 pagesPhase Transformation in AISI 410 Stainless Steel PDFirajfarjiNo ratings yet
- Examen 2 Materiales PasadoDocument5 pagesExamen 2 Materiales PasadoAndres RicoNo ratings yet
- Assignment Metal and MetallurlgyDocument4 pagesAssignment Metal and MetallurlgyGURPRATAP SINGHNo ratings yet
- Master In Metallurgy And Materials Science TALLER N°2Document7 pagesMaster In Metallurgy And Materials Science TALLER N°2ricardo alfonso paredes roaNo ratings yet
- EMM LectureDocument38 pagesEMM Lecturelatendra kumar srivastavNo ratings yet
- CH 11Document9 pagesCH 11Muchammad FauziNo ratings yet
- Heat Treatment of 1045 Steel PDFDocument17 pagesHeat Treatment of 1045 Steel PDFH_DEBIANENo ratings yet
- TTT DiagramDocument2 pagesTTT Diagramthe guy behind the timesNo ratings yet
- Fracture Dependence On Heat Treatment: Table 1-Dependence of Absorbed Energy (For Crack Propagation) Over TemperatureDocument4 pagesFracture Dependence On Heat Treatment: Table 1-Dependence of Absorbed Energy (For Crack Propagation) Over Temperaturelokomoko1No ratings yet
- Handout Chapter 5 Iron Carbon SystemDocument7 pagesHandout Chapter 5 Iron Carbon SystemBikram MuduliNo ratings yet
- MEB 1211 QuestionsDocument8 pagesMEB 1211 QuestionsRaj RajendranNo ratings yet
- Structural SteelsDocument5 pagesStructural SteelsijazNo ratings yet
- Hardening From The Liquid StateDocument5 pagesHardening From The Liquid StateSinhrooNo ratings yet
- E45L - Properties of Materials Laboratory GuideDocument14 pagesE45L - Properties of Materials Laboratory GuidenickNo ratings yet
- SCEP UserGuideDocument25 pagesSCEP UserGuidenickNo ratings yet
- E45 Laboratory6Document8 pagesE45 Laboratory6nickNo ratings yet
- E45 Laboratory6Document8 pagesE45 Laboratory6nickNo ratings yet
- E45 Laboratory5Document6 pagesE45 Laboratory5nickNo ratings yet
- Laboratory 0: Engineering Ethics: ObjectivesDocument2 pagesLaboratory 0: Engineering Ethics: ObjectivesnickNo ratings yet
- E45 Laboratory3Document11 pagesE45 Laboratory3nickNo ratings yet
- E45 Laboratory2Document6 pagesE45 Laboratory2nickNo ratings yet
- The Case of The Cooling CadaverDocument5 pagesThe Case of The Cooling CadavernickNo ratings yet
- E45 Laboratory1Document10 pagesE45 Laboratory1nickNo ratings yet
- Laboratory 0: Engineering Ethics: ObjectivesDocument2 pagesLaboratory 0: Engineering Ethics: ObjectivesnickNo ratings yet
- Optimize Process Flowsheet for Carbonate-Chloride ProcessDocument14 pagesOptimize Process Flowsheet for Carbonate-Chloride ProcessnickNo ratings yet
- Arta Fridei Kahlo A Fost Intotdeauna o ReactieDocument13 pagesArta Fridei Kahlo A Fost Intotdeauna o ReactieAlta DaianNo ratings yet
- CBSE Worksheet-01 Class - VI Science (The Living Organisms and Their Surroundings)Document3 pagesCBSE Worksheet-01 Class - VI Science (The Living Organisms and Their Surroundings)Ushma PunatarNo ratings yet
- Civil ServiceDocument46 pagesCivil ServiceLester Josh SalvidarNo ratings yet
- Radar PPNDocument5 pagesRadar PPNSawaf MfNo ratings yet
- ATEX Certified FiltersDocument4 pagesATEX Certified FiltersMarco LoiaNo ratings yet
- Weber Grills - FinalDocument12 pagesWeber Grills - FinalDIVYANSHU SHEKHARNo ratings yet
- Sensor Controlled Animatronic Hand: Graduation Project PresentationDocument24 pagesSensor Controlled Animatronic Hand: Graduation Project PresentationAnonymous D2FmKSxuuNo ratings yet
- Qand ADocument5 pagesQand AJoshua PascasioNo ratings yet
- Biomechanics of The Knee During Closed Kinetic Chain and Open KineticDocument17 pagesBiomechanics of The Knee During Closed Kinetic Chain and Open KineticArmando NetoNo ratings yet
- Kerala Electricity Regulatory Commission Schedule of TariffDocument36 pagesKerala Electricity Regulatory Commission Schedule of TariffvjtheeeNo ratings yet
- Understanding Earth's History Through Rock CharacteristicsDocument1 pageUnderstanding Earth's History Through Rock CharacteristicsSharmaine AcNo ratings yet
- Mahle KFWA MAIN Data SheetDocument4 pagesMahle KFWA MAIN Data SheetRudnikNo ratings yet
- History: Ludwig Hunger: About Us: Home - Ludwig Hunger GMBHDocument3 pagesHistory: Ludwig Hunger: About Us: Home - Ludwig Hunger GMBHPatrizio MassaroNo ratings yet
- Combustion Cat 2008Document32 pagesCombustion Cat 2008Miguel LinaresNo ratings yet
- Contact GRRSB Team for InquiriesDocument2 pagesContact GRRSB Team for Inquiriesmsis81No ratings yet
- Fairs in Punjab 2021-22Document9 pagesFairs in Punjab 2021-22Suchintan SinghNo ratings yet
- Pentecostal Ecclesiology: Simon K.H. Chan - 978-90-04-39714-9 Via Free AccessDocument156 pagesPentecostal Ecclesiology: Simon K.H. Chan - 978-90-04-39714-9 Via Free AccessStanley JohnsonNo ratings yet
- Template EbcrDocument7 pagesTemplate EbcrNoraNo ratings yet
- Sony HCD-GTX999 PDFDocument86 pagesSony HCD-GTX999 PDFMarcosAlves100% (1)
- P&id BoilerDocument1 pageP&id BoilerBagus AryowibowoNo ratings yet
- Dimensional Analysis Similarity Lesson2 Dimensional Parameters HandoutDocument11 pagesDimensional Analysis Similarity Lesson2 Dimensional Parameters HandoutRizqi RamadhanNo ratings yet
- Mycbseguide: Cbse Class 10 Social Science Sample Paper - 08 (MCQ Based)Document10 pagesMycbseguide: Cbse Class 10 Social Science Sample Paper - 08 (MCQ Based)Abdul MuqsitNo ratings yet
- IMRAD - G1 PepperDocument13 pagesIMRAD - G1 PepperRomero, Ken Angelo B.No ratings yet
- Tec Relay 52GDocument3 pagesTec Relay 52Gimmer nainggolanNo ratings yet
- Knowing Annelida: Earthworms, Leeches and Marine WormsDocument4 pagesKnowing Annelida: Earthworms, Leeches and Marine WormsCherry Mae AdlawonNo ratings yet
- Navmesh Plus: How ToDocument7 pagesNavmesh Plus: How TobladimirNo ratings yet