Professional Documents
Culture Documents
Pulmonary Balantidium Coli Infection in A Leukemic Patient
Uploaded by
Nika Dwi AmbarwatiOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Pulmonary Balantidium Coli Infection in A Leukemic Patient
Uploaded by
Nika Dwi AmbarwatiCopyright:
Available Formats
American Journal of Hematology 73:180183 (2003)
Pulmonary Balantidium coli Infection in a
Leukemic Patient
K. Anargyrou,1 G.L. Petrikkos,2 M.T.E. Suller,3* A. Skiada,2 M.P. Siakantaris,1
R.T. Osuntoyinbo,2 G. Pangalis,1 and G. Vaiopoulos1
1
Hematology Section, 1st Department of Internal Medicine, Athens University School of Medicine, Laikon General Hospital, Goudi,
Athens, Greece
Infectious Diseases and Antimicrobial Chemotherapy Research Laboratory G.K. Daikos, 1st Department of Propaedeutic Medicine,
Athens University School of Medicine, Laikon General Hospital, Goudi, Athens, Greece
3
Faculty of Applied Biosciences, University of the West of England, Frenchay Campus, Bristol, United Kingdom
A 59-year-old woman suffering from chronic lymphocytic leukemia developed pulmonary
lesions; bronchoalveolar lavage was performed for possible systemic fungal infection.
However, direct microscopic analysis revealed ciliated protozoa identified as Balantidium
coli. B. coli is the only known pathogenic ciliate, and is usually associated with intestinal
infection in areas associated with pig rearing. On very rare occasions the organisms may
invade extra-intestinal organs, in this case the lungs of an immunocompromised patient.
This case is unusual as balantidiasis is rare in Europe, the patient had no obvious contact
with pigs, and there was no history of diarrhea prior to pulmonary colonization. Metronidazole was rapidly administered, and the condition improved after 2448 hr. Am. J.
2003 Wiley-Liss, Inc.
Hematol. 73:180183, 2003.
Key words: Balantidium coli; pulmonary; leukemia; parasite
CASE HISTORY
A 58-year-old female was diagnosed with chronic
lymphocytic leukemia, Rai stage 0, in September 1997.
The complete blood cell count (CBC) on diagnosis was:
white blood cells 8 109/l (18% neutrophils, 70% lymphocytes, 7% monocytes, 5% eosinophils), hemoglobin
12.5 g/dl, hematocrit 39.5%, platelets 256 109/l.
The patients condition remained stable until November 1999, when neck, axillary, and inguinal lymphadenopathy developed. The CBC was as follows: white
blood cells 61.2 109/l (10% neutrophils, 87% lymphocytes, 1% monocytes, 2% eosinophils), hemoglobin 11.7
g/dl, hematocrit 36.5%, platelets 160 109/l.
In January 2000, because of the lymphadenopathy and
increased lymphocytes, antileukemic therapy with chlorambucil and methylprednisolone (32 mg/day) was initiated in another hospital. She received a total of 7 cycles
of chlorambucil, at a dose of 10 mg per day, for 14 days
per month, without any significant improvement, either
in terms of lymph nodes or CBC. In December 2000,
fludarabine was administered, at a dose of 30 mg/day, for
5 days. The result of the first cycle was severe pancytopenia, and it was interrupted. The patient chose to leave
the center where she was being treated, and she referred
2003 Wiley-Liss, Inc.
herself to our hematological center. At that time she was
taking only prednisolone (40 mg/day). In January 2001 a
neck lymph node was resected for biopsy. The sections
showed effacement of the normal lymph node architecture by a diffuse population of small lymphocytes, within
which there were small, rather indistinct, clusters of
larger cells with nucleoli. The appearance was consistent
with the diagnosis of small lymphocytic lymphoma/BCLL. Bone marrow biopsy showed a markedly hypercellular marrow with a dense interstitial infiltrate of small
lymphocytes similar to those seen in the lymph node.
Myelopoiesis was seen on small clusters of cells adjacent
to the bone, but myelopoietic reserve was much reduced.
Erythroid cells were difficult to identify. Cell marker
*Correspondence to: Dr. Marc T.E. Suller, Faculty of Applied Biosciences, University of the West of England, Frenchay Campus, Coldharbour Lane, Bristol BS16 1QY, United Kingdom. E-mail:
msuller@lycos.com
Received for publication 24 July 2002; Accepted 15 March 2003
Published online in Wiley InterScience (www.interscience.wiley.com).
DOI: 10.1002/ajh.10336
Case Report: Leukemic Balantidium coli Infection
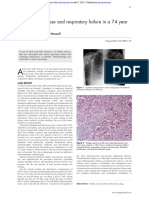
Fig. 1. Chest X ray showing bilateral, diffuse micronodular,
and interstitial lesions in the lungs.
studies showed a typical CLL immunophenotype
(CD19+/CD5+, CD23+, FMC7).
In March 2001 she developed low-grade fever of
37.8C and was treated, at home, with ciprofloxacin, 1
g/day, for 8 days. She remained febrile and in the week
prior to admission to the hospital (16th23rd May 2001),
the patient developed a high-fever of 39.9C with rigors
and a headache that did not respond to analgesics. She
also complained of weakness, weight loss (23 kg in 1
month), abdominal cramps and bloating, a persistent,
nonproductive cough, and dyspnea.
On May 23 she was admitted to our hospital. The
patient was pale and in distress. Her blood pressure was
110/60 mmHg, the pulse rate was 120/min and her temperature 36.6C. Arterial blood gases were as follows:
pO2 42.7 mmHg, pCO2 31.6 mmHg, pH 7.5, HCO3 24.9
mmol/l, saturation 83.3%. Enlarged lymph nodes were
palpated in the cervical, axillary, and inguinal areas. Examination of the head and neck was otherwise normal.
Physical examination of the respiratory and cardiovascular system revealed no significant findings. The abdomen
was soft and nontender, with no palpable organomegaly,
with normal sounds. Her laboratory blood tests were as
follows: hemoglobin 10.5 g/dl, hematocrit 33.8%, white
blood count 28600/l (polymorphonuclear leucocytes
3%, lymphocytes 97%), platelets 84000/l, urea 38 mg/
dl, creatinine 0.9 mg/dl, sodium 138 mEq/l, potassium
4.4 mEq/l, AST 50 U/l, ALT 22 U/l, lactic dehydrogenase 1,344 U/l. A chest X ray showed bilateral, diffuse
micronodular and interstitial lesions in the lungs (Fig. 1).
A chest CT-scan showed enlarged lymph nodes of the
mediastinum, on the right, by the trachea and on the left,
181
near the aortic arch. The axillary lymph nodes were also
enlarged. Bilateral, diffuse micronodular, and interstitial
lesions were seen in the lungs. An abdominal CT scan
showed small enlargement of the liver and spleen, with
no focal lesions, and enlarged para-aortic lymph nodes.
Pancreas and kidneys were normal.
Treatment with ceftriaxone, 2 g/day, and amikacin, 1
g/day, was started. In view of the findings, a fungal (including Pneumocystis carinii) infection of the lung was
suspected, and subsequently a bronchoalveolar lavage
was performed. Direct microscopic examination of the
specimen revealed numerous protozoa that were 50 m
or more in diameter. They had an outer membrane covered by short cilia and a single large kidney-bean-shaped
nucleus. They exhibited a rotary/boring motility, which
became more vigorous with continued illumination. They
were identified as Balantidium coli. Trying to identify a
possible source of infection, multiple questions were
asked, but the patient confirmed that she lived only in
Athens, denied any contact with pigs or other mammals,
and her diet did not include any raw meat.
A stool sample was obtained from the patient which
was nondiarrheal and nonmucoid in appearance, and no
parasites were observed microscopically. Stains and PCR
for P. carinii were repeatedly negative. The patient was
treated for the parasitic infection with metronidazole at
750 mg, administered intravenously 3 times a day for 15
days. After 24 hr the patient was afebrile with a normal
body temperature, and upon the completion of antiparasitic therapy, X rays and CT scans revealed that the lung
lesions had receded (Fig. 2). During the next 6 months
there was no clinical or radiological relapse of the infection, and this led us to the conclusion that the organism
was effectively eliminated. Following the infection, the
patient was again treated with fludarabine, at a reduced
dose, 25 mg/day/month for 1 cycle and then continued
with COP (cyclophosphamide, Oncovin, prednisolone).
DISCUSSION
B. coli is the largest protozoan parasite and the only
ciliated protozoa known to infect humans. Human infection is relatively rare and occurs mainly in tropical regions, especially Latin America, the Far East, and New
Guinea, usually associated with areas of pig rearing [1].
B. coli colonizes the intestine of the pig and then multiplies by asexual reproduction, during which each parasitic cell divides into two daughter cells, by transverse
binary fission. However, the trophozoite will encyst as it
passes through the intestine and is excreted in feces. A
definitive host may ingest the encysted organism and
consequently become infected after de-encystation in the
stomach. Although the pig is the major host for B. coli,
the organism is capable of adapting to new host species,
such as humans, guinea pigs, and other mammals, and
182
Case Report: Anargyrou et al.
Fig. 2. Chest X ray after treatment with metronidazole at
750 mg, administered intravenously 3 times a day for 15
days, revealing that the lung lesions had receded.
cause serious disease. Human balantidiosis is most
prevalent where pigs are raised and slaughtered. Colonization is usually noninvasive and asymptomatic but may
lead to acute or chronic diarrhea [2]. Proteolytic enzymes, e.g., hyaluronidases, are produced that can break
down the intestinal epithelium, resulting in invasion of
the mucosa and colon ulceration [3]. The production of
these proteolytic enzymes might be the factor that differentiates B. coli from other ciliate protozoa, allowing it
to infect humans. As a result, hemorrhaging and secondary bacterial infections may occur. Occasionally, the organisms will perforate the large intestine and affect the
small intestine, [4] the appendix, [3] the vagina, uterus,
and bladder, [5] and on very rare occasions the liver [6]
and lungs [79]. Currently, precise information about
exact morbidity and mortality rates of balantidiasis is
lacking. A study performed in Amerindian populations of
Amazonia [10] found both a low prevalence of the disease and absence of symptoms or signs referable to this
organism. In another study, performed in San Cayetano,
Corrientes, Argentina, 207 stool samples were collected
from children and examined; 83% contained one or more
parasites. The prevalence of B. coli was 0.5% [11]. Immunologic factors, such as IgA and T-cell responses, are
important for giardiasis and spore-forming protozoa, but
there are no studies regarding B. coli and immunosuppression. There is little information about balantidiasis in
HIV-infected patients. In parts of the world where pigs
are the principal domestic animals and standards of hy-
giene are suboptimal, infection rates of Balantidium in
the population can be high, and it is likely that immunodeficiency can lead to exacerbation of this disease. A
case of co-infection by B. coli and HIV has been reported
from French Guiana [12].
Diagnosis of B. coli is established by observing trophozoites in stool or tissue samples. It is relatively easy
to recognize in clinical specimens due to its large size
(50100 m long and 4070 m wide), an outer membrane covered by short cilia and a single large kidneybean-shaped nucleus. In wet mount preparations, it exhibits a rotary/boring motility, and the macronucleus may
be apparent. Iodine may be used to stain the organism,
and the nucleus becomes clearly visible (other staining).
There are several drugs that can be used in the treatment
of balantidiasis: tetracycline, 500 mg qid, for 10 days;
metronidazole, 750 mg tid, for 5 days; or iodoquinol, 650
mg tid, for 20 days [13]. In this case, metronidazole was
chosen because it can be given intravenously and it was
readily available
This case is unusual for three reasons: (i) The incidence of the disease in Europe is very low, so physicians
may not have considered the possibility of balantidiasis.
Several interesting articles, originating in the U.S. [14
16], include discussions about B. coli, but there are no
similar articles from Europe. (ii) B. coli infection in humans is usually associated with poor sanitation and close
proximity to pigs. However, there was no evidence to
suggest any interaction of this patient with pigs. Ladas et
al. [8] reported a balantidium infection of the lungs and
the intestine of a 70-year-old Greek male with bronchial
asthma, who was not immunocompromised, and who
also claimed to have had no contact with pigs. (iii) There
was no history of bloody or mucoid diarrhea leading up
to the diagnosis of balantidiasis, although invasion of the
intestinal mucosa does not always lead to ulceration [8].
There are three other reports of lung balantidiasis in the
literature. The most common site of infection is the intestine. A possible mechanism for lung infection could be
initial colonization of the intestine, ulceration, and subsequent hematological dissemination, although this hypothesis remains to be proven. In our case diagnosis was
established as a consequence of the results of the CT
scan, which revealed lesions in the lungs. As the patient
was suffering from leukemia, and thus was immunocompromised, a systemic mold infection was suspected. For
this reason, a BAL specimen was collected, in which the
parasite was observed microscopically. Without rapid
initiation of therapy, the patients condition would probably have rapidly deteriorated. Treatment with the appropriate antibiotic (metronidazole) and rapid improvement confirm the diagnosis of B. coli as the etiology of
the febrile pulmonary disease of this immunocompromised patient. The patient was immunocompromised
from having leukemia, from being on long-term cortico-
Case Report: Leukemic Balantidium coli Infection
steroids, and from receiving chemotherapy, which affects
lymphocyte function. It would seem logical to conclude
that the above factors played a role in the infection, but
no studies about balantidiasis in immunocompromised
patients exist. Parasites pose a serious problem in the
patient with impaired lymphocyte function, and further
studies are needed to elucidate their mode of action.
REFERENCES
1. Radford AJ. Balantidiasis in Papua New Guinea. Med J Aust 1973;1:
238241.
2. Swartzwelder JC. Balantidiasis. Am J Dig Dis 1950;17:173179.
3. Arean VM, Koppisch E. Balantidiasis: a review and report of cases.
Am J Pathol 1956;32:10891116.
4. Baskerville L, Ahmed Y, Ramchad S. Balantidium colitis. Am J Dig
Dis 1970;15:727731.
5. Knight R. Giardiasis, isosporiasis and balantidiasis. Clin Gastoenterol
1978;7:3147.
6. Wegner F. Abscesso hepatico producido por el Balantidium coli. Casemera 1967;2:433441.
7. Dorfman S, Rangel O, Bravo LG. Balantidiasis: a report of a fatal case
8.
9.
10.
11.
12.
13.
14.
15.
16.
183
with appendicular and pulmonary involvement. Trans R Soc Trop Med
Hyg 1984;78:833835.
Ladas SD, Sava S, Frydas A, et al. Invasive balantidiasis presented as
chronic colitis and lung involvement. Dig Dis Sci 1989;34:16211623.
OConner C, Sharma S. Balantidium coli infection of lungs: a first case
report. Am J Resp Crit Care Med 1999;34:A298.
Lawrence DN, Neel JV, Abadie SH, Moore LL, Adams LJ, Healy GR,
Kagan IG. Epidemiologic studies among Amerindian populations of
Amazonia. III. Intestinal parasitoses in newly contacted and acculturating villages. Am J Trop Med Hyg 1980;29(4):530537.
Borda CE, Rea MJ, Rosa JR, et al. Intestinal parasitism in San Cayetano, Corrientes, Argentina. Bull Pan Am Health Org 1996;30(3):
227233.
Clyti E, Aznar C, Couppie P, et al. A case of coinfection by Balantidium coli and HIV in French Guiana. Bull Soc Pathol Exot 1998;
91(4):309311.
Drugs for parasitic infections. The Medical Letter on Drugs and Therapeutics. New Rochelle: The Medical Letter, Inc.; 2002.
Weiss LM, Keohane EM. The uncommon gastrointestinal protozoa:
Microsporidia, Blastocystis, Isospora, Dientamoeba, and Balantidium.
Curr Clin Top Infect Dis 1997;17:147187.
Garcia LS. Flagellates and ciliates. Clin Lab Med 1999;19(3):621
638, vii.
Juckett G. Intestinal protozoa. Am Fam Phys 1996;53(8):25072518.
You might also like
- Diversity and InclusionDocument23 pagesDiversity and InclusionJasper Andrew Adjarani80% (5)
- Case 37-2020: A 35-Year-Old Man With Lymphadenopathy and PetechiaeDocument11 pagesCase 37-2020: A 35-Year-Old Man With Lymphadenopathy and PetechiaePatriciaNo ratings yet
- North American Indians - A Very Short IntroductionDocument147 pagesNorth American Indians - A Very Short IntroductionsiesmannNo ratings yet
- Larry Dossey - HealingBeyondtheBodyDocument2 pagesLarry Dossey - HealingBeyondtheBodypaulxeNo ratings yet
- Actinomyces OdontolyticusDocument4 pagesActinomyces OdontolyticusLiviu Athos TamasNo ratings yet
- Pulmonary Miliary Tuberculosis Complicated With Tuberculous Spondylitis An Extraordinary Rare Association: A Case ReportDocument5 pagesPulmonary Miliary Tuberculosis Complicated With Tuberculous Spondylitis An Extraordinary Rare Association: A Case ReportIsmail Eko SaputraNo ratings yet
- Malignant Mesothelioma Presenting With Unexplained Recurrent Pleurisy EpisodesDocument4 pagesMalignant Mesothelioma Presenting With Unexplained Recurrent Pleurisy EpisodesNurul NingrumNo ratings yet
- Staphy 2Document5 pagesStaphy 2Alexandra MariaNo ratings yet
- Case Report: Streptococcus AnginosusDocument4 pagesCase Report: Streptococcus AnginosusIesanu MaraNo ratings yet
- Jurnal Balantidium Coli PDFDocument6 pagesJurnal Balantidium Coli PDFNika Dwi AmbarwatiNo ratings yet
- Adult Still's Disease and Respiratory Failure in A 74 Year Old WomanDocument3 pagesAdult Still's Disease and Respiratory Failure in A 74 Year Old WomanEduardo Romero StéfaniNo ratings yet
- Crim Em2013-948071Document2 pagesCrim Em2013-948071m.fahimsharifiNo ratings yet
- Multiple Episodes of Hypoglycemia Secondary To An Insulinoma Case ReportDocument4 pagesMultiple Episodes of Hypoglycemia Secondary To An Insulinoma Case ReportmiguelNo ratings yet
- Manuscript Case Report Addison's Disease Due To Tuberculosis FinalDocument22 pagesManuscript Case Report Addison's Disease Due To Tuberculosis FinalElisabeth PermatasariNo ratings yet
- Barth SyndromeDocument4 pagesBarth SyndromeC_DanteNo ratings yet
- Pediatric Round PneumoniaDocument4 pagesPediatric Round PneumoniaSalwa Zahra TsamaraNo ratings yet
- 2000 - Frankel - Re Radical Prostatectomy For Localized Prostate Cancer Provides Durable Cancer Control With Excellent Quality of Life A STRDocument2 pages2000 - Frankel - Re Radical Prostatectomy For Localized Prostate Cancer Provides Durable Cancer Control With Excellent Quality of Life A STRPoljarLijanNo ratings yet
- Scientific Letters: WWW - Elsevier.es/eimcDocument2 pagesScientific Letters: WWW - Elsevier.es/eimcfeby1992No ratings yet
- Landouzy Septicemia (Sepsis Tuberculosa Acutissima) Due To Mycobacterium Microti in An Immunocompetent ManDocument4 pagesLandouzy Septicemia (Sepsis Tuberculosa Acutissima) Due To Mycobacterium Microti in An Immunocompetent Manreadyboy89No ratings yet
- 260-Article Text-875-1-10-20220728Document4 pages260-Article Text-875-1-10-20220728hasan andrianNo ratings yet
- Hiding in The Water: Clinical Problem-SolvingDocument6 pagesHiding in The Water: Clinical Problem-SolvingtabareeNo ratings yet
- Rodgers 2015Document5 pagesRodgers 2015No LimitNo ratings yet
- Journal of Medical Case ReportsDocument5 pagesJournal of Medical Case ReportsDumitru RadulescuNo ratings yet
- Could COVID-19 Induce Remission of Acute LeukemiaDocument5 pagesCould COVID-19 Induce Remission of Acute LeukemiaAhmed RichiNo ratings yet
- Tuberculosis of The Chest Wall With Massive Tuberculous Pleural EffusionDocument3 pagesTuberculosis of The Chest Wall With Massive Tuberculous Pleural EffusionwulanNo ratings yet
- Human Brucellosis: John M. Sauret, MD, and Natalia Vilissova, MDDocument6 pagesHuman Brucellosis: John M. Sauret, MD, and Natalia Vilissova, MDAndika Budi KurniantoNo ratings yet
- Ofy 038Document5 pagesOfy 038Rosandi AdamNo ratings yet
- Disseminated Tuberculosis in An AIDS/HIV-Infected Patient: AbstractDocument3 pagesDisseminated Tuberculosis in An AIDS/HIV-Infected Patient: AbstractAmelia Fitria DewiNo ratings yet
- Delay in Diagnosis of Extra-Pulmonary Tuberculosis by Its Rare Manifestations: A Case ReportDocument5 pagesDelay in Diagnosis of Extra-Pulmonary Tuberculosis by Its Rare Manifestations: A Case ReportSneeze LouderNo ratings yet
- Recurrent Pneumonia Caused by Rare Granular Cell TumorDocument7 pagesRecurrent Pneumonia Caused by Rare Granular Cell TumorJoshua MendozaNo ratings yet
- 2013 1 5 PDFDocument2 pages2013 1 5 PDF04lubna_869632400No ratings yet
- Background: Ormdl3 and of GSDMB Were Significantly Increased in Hrv-Stimulated PBMCSDocument6 pagesBackground: Ormdl3 and of GSDMB Were Significantly Increased in Hrv-Stimulated PBMCSSav GaNo ratings yet
- Isolated Mediastnal TuberculosisDocument2 pagesIsolated Mediastnal TuberculosisRiski DohartuaNo ratings yet
- Jurnal Bronchitis Dengan AsmaDocument5 pagesJurnal Bronchitis Dengan AsmaMauLan SaputraNo ratings yet
- 3a. FODDocument3 pages3a. FODRaida Uceda GarniqueNo ratings yet
- 3a. FODDocument3 pages3a. FODRaida Uceda GarniqueNo ratings yet
- Pathology BDocument5 pagesPathology Bttdjhg2p6kNo ratings yet
- Atypical Presentation of Pseudomembranous Colitis Localized in Adenomatous PolypsDocument3 pagesAtypical Presentation of Pseudomembranous Colitis Localized in Adenomatous PolypsKiana TehraniNo ratings yet
- Pi Is 0016508500545053Document5 pagesPi Is 0016508500545053Abdullah KhanNo ratings yet
- Pi Is 0016508500545053Document5 pagesPi Is 0016508500545053Abdullah KhanNo ratings yet
- Chronic Malabsorption Due Child With Immunoglobulin DeficiencyDocument3 pagesChronic Malabsorption Due Child With Immunoglobulin DeficiencyCentro De Copiado Luna RtNo ratings yet
- Yersinia Enterocolitica: A Rare Cause of Infective EndocarditisDocument3 pagesYersinia Enterocolitica: A Rare Cause of Infective EndocarditisDIEGO FERNANDO TULCAN SILVANo ratings yet
- Microbiology Infectious Disease Case StudiesDocument18 pagesMicrobiology Infectious Disease Case StudiesKayeNo ratings yet
- Case Report and Literature Review On Good's Syndrome, A Form of Acquired Immunodeficiency Associated With ThymomasDocument7 pagesCase Report and Literature Review On Good's Syndrome, A Form of Acquired Immunodeficiency Associated With ThymomasMudassar SattarNo ratings yet
- Tuberculoma of the Brain: Rare Case of Multiple Lesions and Meningeal PlaquesDocument9 pagesTuberculoma of the Brain: Rare Case of Multiple Lesions and Meningeal PlaquessanatthhNo ratings yet
- 56 SyedDocument3 pages56 SyededitorijmrhsNo ratings yet
- A Fatal Case of Fungemia and Pneumonia in A Kidney Transplant Recipient During Caspofungin TreatmentDocument20 pagesA Fatal Case of Fungemia and Pneumonia in A Kidney Transplant Recipient During Caspofungin TreatmentLazimatul KhaqNo ratings yet
- Case Report: A Case Report of Peritoneal Tuberculosis: A Challenging DiagnosisDocument4 pagesCase Report: A Case Report of Peritoneal Tuberculosis: A Challenging DiagnosisDumitru RadulescuNo ratings yet
- Treatment of Refractory Pyoderma Gangrenosum with Infliximab in a 17-Month-Old BoyDocument6 pagesTreatment of Refractory Pyoderma Gangrenosum with Infliximab in a 17-Month-Old BoyJares Clinton Saragih SimarmataNo ratings yet
- Early - Stage Hodgkins Lymphoma in A Child A CaseDocument6 pagesEarly - Stage Hodgkins Lymphoma in A Child A CaseХарш Навнітбхаі НімаватNo ratings yet
- Methimazole Drug Induced Agranulocytosis DefinitionDocument21 pagesMethimazole Drug Induced Agranulocytosis DefinitionReyza ReyzaNo ratings yet
- Carcinogen It yDocument8 pagesCarcinogen It yStephanie MartinNo ratings yet
- Podostroma Cornu-Damae Intoxication Induced Pancytopenia and Skin Desquamation Successfully Treated with G-CSFDocument5 pagesPodostroma Cornu-Damae Intoxication Induced Pancytopenia and Skin Desquamation Successfully Treated with G-CSF김성현No ratings yet
- J Clin Pathol 1980 Brook 1099 101Document4 pagesJ Clin Pathol 1980 Brook 1099 101ysh_girlNo ratings yet
- Jurnal 7 PulmoDocument7 pagesJurnal 7 Pulmomisra lailaNo ratings yet
- Case Report: Severe Hyperbilirubinemia: A Rare Complication of Lyme DiseaseDocument4 pagesCase Report: Severe Hyperbilirubinemia: A Rare Complication of Lyme DiseaseDhruva PatelNo ratings yet
- AteneoDocument8 pagesAteneofabiandionisioNo ratings yet
- IJRP 100931120222804 (1) - CompressedDocument7 pagesIJRP 100931120222804 (1) - CompressedKlinik Bpjs AthariNo ratings yet
- Acute Epiglottitis in The Immunocompromised Host: Case Report and Review of The LiteratureDocument5 pagesAcute Epiglottitis in The Immunocompromised Host: Case Report and Review of The Literaturerosandi sabollaNo ratings yet
- Unusual Case of Tuberculous Pleural Effusion and Abdominal MassDocument4 pagesUnusual Case of Tuberculous Pleural Effusion and Abdominal MassRofi IrmanNo ratings yet
- Coexistence of Cutaneous Tuberculosis (Scrofuloderma) and Hanseniasis-A Rare PresentationDocument2 pagesCoexistence of Cutaneous Tuberculosis (Scrofuloderma) and Hanseniasis-A Rare PresentationGesza Utama PutraNo ratings yet
- Corynebacterium Diphtheriae. Other Corynebacterium Species Can Be Responsible, But This Is RareDocument7 pagesCorynebacterium Diphtheriae. Other Corynebacterium Species Can Be Responsible, But This Is RareJocelyn Punzalan AndresNo ratings yet
- Interleukins in Cancer Biology: Their Heterogeneous RoleFrom EverandInterleukins in Cancer Biology: Their Heterogeneous RoleRating: 5 out of 5 stars5/5 (1)
- Diphtheria Prevention at Puskesmas Langensari 1Document2 pagesDiphtheria Prevention at Puskesmas Langensari 1Nika Dwi AmbarwatiNo ratings yet
- Print Flebo+imserDocument3 pagesPrint Flebo+imserNika Dwi AmbarwatiNo ratings yet
- Print Flebo+imserDocument3 pagesPrint Flebo+imserNika Dwi AmbarwatiNo ratings yet
- Project Overview PresentationDocument11 pagesProject Overview PresentationHugo Chorão PereiraNo ratings yet
- Jurnal Balantidium Coli PDFDocument6 pagesJurnal Balantidium Coli PDFNika Dwi AmbarwatiNo ratings yet
- Pyrolysis: Mathematical Modeling of Hydrocarbon Pyrolysis ReactionsDocument8 pagesPyrolysis: Mathematical Modeling of Hydrocarbon Pyrolysis ReactionsBahar MeschiNo ratings yet
- GNED 500 Social AnalysisDocument2 pagesGNED 500 Social AnalysisEshita SinhaNo ratings yet
- Touratsoglou, Coin Production and Circulation in Roman Peloponesus PDFDocument23 pagesTouratsoglou, Coin Production and Circulation in Roman Peloponesus PDFCromwellNo ratings yet
- Identifying States of Matter LessonDocument2 pagesIdentifying States of Matter LessonRaul OrcigaNo ratings yet
- Ass 3 MGT206 11.9.2020Document2 pagesAss 3 MGT206 11.9.2020Ashiqur RahmanNo ratings yet
- So Neither or NorDocument2 pagesSo Neither or NorMita KusniasariNo ratings yet
- HandoverDocument23 pagesHandoveryekoyesewNo ratings yet
- John R. Van Wazer's concise overview of phosphorus compound nomenclatureDocument7 pagesJohn R. Van Wazer's concise overview of phosphorus compound nomenclatureFernanda Stuani PereiraNo ratings yet
- SLE On TeamworkDocument9 pagesSLE On TeamworkAquino Samuel Jr.No ratings yet
- Giles. Saint Bede, The Complete Works of Venerable Bede. 1843. Vol. 8.Document471 pagesGiles. Saint Bede, The Complete Works of Venerable Bede. 1843. Vol. 8.Patrologia Latina, Graeca et Orientalis100% (1)
- Journal of Ethnic Foods: Angelina Rianti, Agnes E. Novenia, Alvin Christopher, Devi Lestari, Elfa K. ParassihDocument6 pagesJournal of Ethnic Foods: Angelina Rianti, Agnes E. Novenia, Alvin Christopher, Devi Lestari, Elfa K. ParassihHerlinaNo ratings yet
- Jason A Brown: 1374 Cabin Creek Drive, Nicholson, GA 30565Document3 pagesJason A Brown: 1374 Cabin Creek Drive, Nicholson, GA 30565Jason BrownNo ratings yet
- Experiment 5 ADHAVANDocument29 pagesExperiment 5 ADHAVANManoj Raj RajNo ratings yet
- SMAW Product DevelopmentDocument9 pagesSMAW Product Developmenttibo bursioNo ratings yet
- New Intelligent AVR Controller Based On Particle Swarm Optimization For Transient Stability EnhancementDocument6 pagesNew Intelligent AVR Controller Based On Particle Swarm Optimization For Transient Stability EnhancementnaghamNo ratings yet
- The Great Idea of Brook TaylorDocument7 pagesThe Great Idea of Brook TaylorGeorge Mpantes mathematics teacherNo ratings yet
- Commonlit Bloody KansasDocument8 pagesCommonlit Bloody Kansasapi-506044294No ratings yet
- Olimpiada Engleza 2017 CL A 7 A PDFDocument4 pagesOlimpiada Engleza 2017 CL A 7 A PDFAnthony Adams100% (3)
- AREVA Directional Over Current Relay MiCOM P12x en TechDataDocument28 pagesAREVA Directional Over Current Relay MiCOM P12x en TechDatadeccanelecNo ratings yet
- SOW Form 4 2017Document8 pagesSOW Form 4 2017ismarizalNo ratings yet
- Case DurexDocument3 pagesCase DurexGia ChuongNo ratings yet
- Advantages and Disadvantages of A MonopolyDocument7 pagesAdvantages and Disadvantages of A MonopolyRosalyn RayosNo ratings yet
- Sons and Lovers AuthorDocument9 pagesSons and Lovers AuthorArmen NeziriNo ratings yet
- Social Marketing PlanDocument25 pagesSocial Marketing PlanChristophorus HariyadiNo ratings yet
- Absolute TowersDocument11 pagesAbsolute TowersSandi Harlan100% (1)
- Assalamu'alaikum WR WB.: Emcee Script (1) Pre - AnnouncementDocument3 pagesAssalamu'alaikum WR WB.: Emcee Script (1) Pre - AnnouncementGian AlfaNo ratings yet
- Basic Statistical Tools for Data Analysis and Quality EvaluationDocument45 pagesBasic Statistical Tools for Data Analysis and Quality EvaluationfarjanaNo ratings yet