Professional Documents
Culture Documents
Graph Discussion
Uploaded by
Honeylet Ü FerolCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Graph Discussion
Uploaded by
Honeylet Ü FerolCopyright:
Available Formats
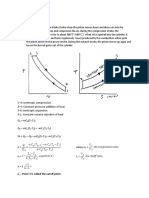
In a typical graph of moisture content versus drying rate and moisture content versus time,
section AB represents the constant-rate period. In that zone, moisture is considered to be evaporating from
a saturated surface at a rate governed by diffusion from the surface through the stationary air film that is
in contact with it. This period depends on the air temperature, humidity and speed of moisture to the
surface, which in turn determine the temperature of the saturated surface. During the constant rate period,
liquid must be transported to the surface at a rate sufficient to maintain saturation.
At the end of the constant rate period, (point B, Figure 1), a break in the drying curve occurs. This
point is called the critical moisture content, and a linear fall in the drying rate occurs with further drying.
This section, segment BC, is called the first falling-rate period. As drying proceeds, moisture reaches the
surface at a decreasing rate and the mechanism that controls its transfer will influence the rate of drying.
Since the surface is no longer saturated, it will tend to rise above the wet bulb temperature. This section,
represented by segment CD in Figure 1 is called the second falling-rate period, and is controlled by vapor
diffusion. Movement of liquid may occur by diffusion under the concentration gradient created by the
depletion of water at the surface. The gradient can be caused by evaporation, or as a result of capillary
forces, or through a cycle of vaporization and condensation, or by osmotic effects.
The capacity of the air (gas) stream to absorb and carry away moisture determines the drying rate
and establishes the duration of the drying cycle. The two elements essential to this process are inlet air
temperature and air flowrate. The higher the temperature of the drying air, the greater its vapor holding
capacity. Since the temperature of the wet granules in a hot gas depends on the rate of evaporation, the
key to analyzing the drying process is psychrometry, defined as the study of the relationships between the
material and energy balances of water vapor and air mixture.
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- Quality Assurance and Quality ControlDocument17 pagesQuality Assurance and Quality Controltraslie0% (1)
- Hazards - Biological, Chemical and PhysicalDocument16 pagesHazards - Biological, Chemical and PhysicalUsman Cheema100% (1)
- Rtwo SamplesDocument1 pageRtwo SamplesHoneylet Ü FerolNo ratings yet
- TheoryDocument7 pagesTheoryHoneylet Ü FerolNo ratings yet
- Date of Analysis Sample Product Sample Code Initial Weight Final Weight Ash Content Sample Product Date of AnalysisDocument1 pageDate of Analysis Sample Product Sample Code Initial Weight Final Weight Ash Content Sample Product Date of AnalysisHoneylet Ü FerolNo ratings yet
- DR Jose RizalDocument1 pageDR Jose RizalHoneylet Ü FerolNo ratings yet
- Strong & Weak Acid Titrations: Equivalence Point pH DifferencesDocument1 pageStrong & Weak Acid Titrations: Equivalence Point pH DifferencesHoneylet Ü FerolNo ratings yet
- InterviewDocument1 pageInterviewHoneylet Ü FerolNo ratings yet
- In This ExperimentDocument1 pageIn This ExperimentHoneylet Ü FerolNo ratings yet
- ManDocument3 pagesManHoneylet Ü FerolNo ratings yet
- Miriam PsycDocument6 pagesMiriam PsycHoneylet Ü FerolNo ratings yet
- Proj PropDocument4 pagesProj PropHoneylet Ü FerolNo ratings yet
- InterviewDocument1 pageInterviewHoneylet Ü FerolNo ratings yet
- Table Exp2Document1 pageTable Exp2Honeylet Ü FerolNo ratings yet
- Date Performed: Feb. 14, 2017 - POLVORONDocument3 pagesDate Performed: Feb. 14, 2017 - POLVORONHoneylet Ü FerolNo ratings yet
- ReferencesDocument1 pageReferencesHoneylet Ü FerolNo ratings yet
- RnaDocument3 pagesRnaHoneylet Ü Ferol100% (1)
- ArticleDocument3 pagesArticleHoneylet Ü FerolNo ratings yet
- Exp 2Document4 pagesExp 2Honeylet Ü FerolNo ratings yet
- Exp 3Document3 pagesExp 3Honeylet Ü FerolNo ratings yet
- IntroDocument1 pageIntroHoneylet Ü FerolNo ratings yet
- Use of Synthetic Dyes ExperimentDocument3 pagesUse of Synthetic Dyes ExperimentHoneylet Ü FerolNo ratings yet
- Accomplishment Report 2Document1 pageAccomplishment Report 2Honeylet Ü FerolNo ratings yet
- Exp 6Document3 pagesExp 6Honeylet Ü FerolNo ratings yet
- DiscussionDocument2 pagesDiscussionHoneylet Ü FerolNo ratings yet
- Exp 1 ADocument2 pagesExp 1 AHoneylet Ü FerolNo ratings yet
- Drying JD Is Waj Di WoowDocument10 pagesDrying JD Is Waj Di WoowHoneylet Ü FerolNo ratings yet
- Pump Brand/ Manufacturer: DAICHI PUMP Model: D-3043 Power: 2.2kW, 3Hp Volume Flow Rate: 0.7m /min Head: 10m Price: PHP 22,999Document3 pagesPump Brand/ Manufacturer: DAICHI PUMP Model: D-3043 Power: 2.2kW, 3Hp Volume Flow Rate: 0.7m /min Head: 10m Price: PHP 22,999Honeylet Ü FerolNo ratings yet
- Effect of heat and pH on vegetable pigmentsDocument1 pageEffect of heat and pH on vegetable pigmentsHoneylet Ü FerolNo ratings yet
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (894)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Diesel Cycle: R - Point 3 Is Called The Cutoff PointDocument7 pagesDiesel Cycle: R - Point 3 Is Called The Cutoff PointJethro Briza GaneloNo ratings yet
- DataSheet - StorMaxx NP Storage TankDocument4 pagesDataSheet - StorMaxx NP Storage TankSunMaxx SolarNo ratings yet
- Concealed Duct Spec Sheet A4 - Print CompressedDocument2 pagesConcealed Duct Spec Sheet A4 - Print CompressedJames HillNo ratings yet
- Psych ExercisDocument2 pagesPsych ExercisJohn Jonel CasupananNo ratings yet
- Chem 1st FinDocument333 pagesChem 1st Finjzjz14324No ratings yet
- Evaporador PDFDocument31 pagesEvaporador PDFAdriano RafaelNo ratings yet
- Environment EconomicsDocument9 pagesEnvironment EconomicsUmar SaleemNo ratings yet
- Lecture34 1Document38 pagesLecture34 1vamsikrishna14No ratings yet
- DUPLEX 500 - 11000 Multi EN - 2018 - 03Document8 pagesDUPLEX 500 - 11000 Multi EN - 2018 - 03Constantin CilibiuNo ratings yet
- SCI212 Lab3 InstructionsDocument2 pagesSCI212 Lab3 InstructionsyvaiynelhauralopezNo ratings yet
- ME City Multi VRF 07-20Document134 pagesME City Multi VRF 07-20John AristaNo ratings yet
- EEE112 Engineering Mechanics and Thermodynamics PECDocument6 pagesEEE112 Engineering Mechanics and Thermodynamics PECAnosh BangashNo ratings yet
- Le ChâtelierDocument17 pagesLe Châteliercacancella21No ratings yet
- Exothermic, Endothermic WebquestDocument2 pagesExothermic, Endothermic Webquestshaylabrack1No ratings yet
- Unit 2-Chapter 4 - Solidification and Phase DiagramsDocument105 pagesUnit 2-Chapter 4 - Solidification and Phase Diagramssainath reddy kesam reddyNo ratings yet
- AIR BLOWERS Calculation-of-Air-Pipe-SizeDocument7 pagesAIR BLOWERS Calculation-of-Air-Pipe-SizeEngFaisal Alrai100% (3)
- Experiment B - Heat Exchanger WorksheetDocument11 pagesExperiment B - Heat Exchanger WorksheetKelvin Chew100% (1)
- Review ProcessDocument31 pagesReview ProcessGuillermo Serralde PaezNo ratings yet
- Liquid Out, Temperature 25.5 °C Tube: M/gs P / WDocument7 pagesLiquid Out, Temperature 25.5 °C Tube: M/gs P / WGianra RadityaNo ratings yet
- CM Brochure Yeke Series Commercial Split SystemsDocument3 pagesCM Brochure Yeke Series Commercial Split SystemsRUMANEY BORNNo ratings yet
- Reactor Design IIDocument68 pagesReactor Design IIKORAMA KIEN0% (1)
- TT7-2.2KC3-2 (Rev 02)Document5 pagesTT7-2.2KC3-2 (Rev 02)Jacenty FrymusNo ratings yet
- Nucleate BoilingDocument376 pagesNucleate Boilingrzlisk011713100% (1)
- Jaegle PDFDocument7 pagesJaegle PDFManikandan SundararajNo ratings yet
- Revamping of The PCS Nitrogen 03 Plant in Trinidad: Elizabeth West-ToolseeDocument7 pagesRevamping of The PCS Nitrogen 03 Plant in Trinidad: Elizabeth West-Toolseevaratharajan g rNo ratings yet
- Applied Thermodynamics-II BTech 7th Mechanical UNIT 1Document6 pagesApplied Thermodynamics-II BTech 7th Mechanical UNIT 1Arjumand MehakNo ratings yet
- Tecumseh Guidelines For Utilization of R600A and R290.AshxDocument4 pagesTecumseh Guidelines For Utilization of R600A and R290.AshxVinod NairNo ratings yet
- TRANSPORT PHENOMENA ASSIGNMENT HEAT TRANSFER PROBLEMSDocument2 pagesTRANSPORT PHENOMENA ASSIGNMENT HEAT TRANSFER PROBLEMSNitish NehraNo ratings yet
- Thermodynamic Properties of R134a (1112-Tetrafluoroethane) PDFDocument11 pagesThermodynamic Properties of R134a (1112-Tetrafluoroethane) PDFJuan Daniel Perez LopezNo ratings yet
- Quizz Chapter 2Document6 pagesQuizz Chapter 2torreblancacamilaNo ratings yet