Professional Documents
Culture Documents
Determination of The Sample Identity
Uploaded by
Tipa LaoOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Determination of The Sample Identity
Uploaded by
Tipa LaoCopyright:
Available Formats
Determination of the Sample Identity
1.2
Relative Intensity
0.8
0.6
0.4
0.2
0
0 20 40 60 80 100
2
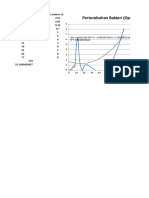
Figure 1 Xray Diffractogram of the "BaTiO3" Sample
*insert jpcds of BaTiO3, BaCO3, and TiO2*
*insert pdf number of these jpcds*
*compared these jpcds with d column of Table 1*
*identify impurities*
*what causes the presence of impurities?*
Conclusion: Therefore, the sintered sample is _________.
***
Determination of the Crystal Structure
Table 1 Determination of Lattice Parameter, a, using the Intensity Peaks from the X-Ray Diffractogram
2 Relative + hkl a () d
Intensity + lattice interplanar
parameter spacing
22.19 0.176629 1 1 100 4.00286 4.002864182
31.57 1 1.99827 2 110 4.00286 2.831677499*
38.92 0.295165 2.997106 3 111 4.00286 2.31217028*
45.34 0.21323 4.011446 4 200 4.00286 1.998574555
51.06 0.063801 5.015883 5 210 4.00286 1.787298741
56.22 0.261921 5.994783 6 211 4.00286 1.634873423*
65.93 0.120887 7.995135 8 220 4.00286 1.415656712
70.47 0.03996 8.98823 9 221 4.00286 1.335161398
75.09 0.068502 10.02786 10 301 4.00286 1.264056934
79.40 0.038617 11.01823 11 311 4.00286 1.205910002
*d spacing of the top three peaks with the highest intensity
(K1): 1.540593
Crystal Structure: Simple Cubic
Why?
The table above showed a 1-2-3-4-5-6-8 h2+k2+l2 pattern.
Figure 2 Phase Transformation of BaTiO3, (Buchanan and Park 1997)
BaTiO3 prefers the cubic phase, (Buchanan and Park, 1997).
Calcination Parameters: 1000C, 5 hours, 5C/min
Regrinding: 47 minutes, 36 seconds
Pelletizing: 1450 psi, 2 min
Sintering Parameters: 1000C, 2 hours, 10C/min
Table 2 h2+k2+l2 Patterns for Cubic Crystal Structures
Crystal Structure h2+k2+l2 Patterns
Simple Cubic 1, 2, 3, 4, 5, 6, 8, 9, 10, 11, 12, 13, 14, 16
Face Centered Cubic 3, 4, 8, 11, 12, 16, 19, 20, 24, 27, 32
Body Centered Cubic 2, 4, 6, 8, 10, 12, 14, 16
Diamond Cubic 3, 8, 11, 16, 19, 24, 27, 32
Conclusion: The crystal structure of BaTiO3 sample was simple cubic, because _________.
***
Determination of Density
Formula Density, g/mL % error
(compared to
theoretical that is
based in the
literature)
Theoretical (literature) (Fisher Scientific, 2015) 6.08 0
Theoretical (thru XRD 6.037596108 0.69743244
=
data)
n: # atoms in the unit cell
MW: molecular weight of
BaTiO3, 233.194 g/mol
NA: Avogadros Number,
6.022 1023
Vc: volume of the unit cell
Experimental (Normal) 2.718680433 55.2848613
=
mair: mass of sample
(suspended in air)
V: calculated volume using the
measured dimensions of the
sample
Experimental (thru 5.514561497 9.299975378
=
Archimedes Principle)
mEtOH: mass of sample
(suspended in ethanol)
Conclusion:
1. In terms of accuracy, XRD method gave a more accurate reading of density. While, the
normal method gave the least accurate density value.
2. Compared to the Archimedes Principle, the Normal Method gave a less accurate value,
because of *insert reason of errors*. Air buoyancy? What?
References
[1] Buchanan, Relva C., and Taeun Park. Materials Crystal Chemistry. New York: Marcel Dekker Inc., 1997.
[2] University of Alabama. "Laboratory Module 1: Indexing Diffraction Patterns from Cubic Materials." n.d.
http://bama.ua.edu/~mweaver/courses/MTE481/Lab1_2009.pdf.
[3] Fisher Scientific. Barium Titanate Safety Data Sheet. February 10, 2015.
https://www.fishersci.com/shop/msdsproxy?productName=AC196865000&productDescription=BARIUM+TITANAT
E(IV)%2C+99%25+500GR&catNo=AC19686-5000&vendorId=VN00032119&storeId=10652 (accessed November 4,
2015).
You might also like
- AssignmentDocument4 pagesAssignmentBakhita MaryamNo ratings yet
- Mlr-Ii Practical 4.3Document3 pagesMlr-Ii Practical 4.3Pooja ChauhanNo ratings yet
- Amiel A. Cabatchete Bsce-4B Group No. 1 SEPTEMBER 19, 2017: Activity # 3Document10 pagesAmiel A. Cabatchete Bsce-4B Group No. 1 SEPTEMBER 19, 2017: Activity # 3EmanoAceNo ratings yet
- Group 7 - Data Sheet (Experiment 2)Document12 pagesGroup 7 - Data Sheet (Experiment 2)Jeremy Kyle Edson AustriaNo ratings yet
- Title: X - Ray Analysis of Ceramic Materials. Aim:: N N D IDocument4 pagesTitle: X - Ray Analysis of Ceramic Materials. Aim:: N N D IDimantha SwarnapalaNo ratings yet
- Physics Lab Report - PendulumDocument8 pagesPhysics Lab Report - PendulumRyan SongNo ratings yet
- Team 2, Please Contact Alex Franco (Afrancoh@fiu - Edu) in Advance Regarding Running YourDocument4 pagesTeam 2, Please Contact Alex Franco (Afrancoh@fiu - Edu) in Advance Regarding Running YoursantanucctNo ratings yet
- Stat Activity 2 Group 4Document12 pagesStat Activity 2 Group 4Jireh RiveraNo ratings yet
- Basics of X-Ray Powder DiffractionDocument82 pagesBasics of X-Ray Powder DiffractionJohn Gerald OdhiamboNo ratings yet
- Fadhlina XRD PDFDocument11 pagesFadhlina XRD PDFMuzamir MahatNo ratings yet
- List of Figures: Figur e No. Figure TitleDocument7 pagesList of Figures: Figur e No. Figure TitleAli Mustafa AllafiNo ratings yet
- Report Full Direct Shear Test Edit Repaired PDFDocument15 pagesReport Full Direct Shear Test Edit Repaired PDFarif daniel muhamaddunNo ratings yet
- G11 Practical 8 (B)Document22 pagesG11 Practical 8 (B)CHAN KOON SEANNo ratings yet
- Geotech SHEAR BOX Final Report - MichaelMamattaDocument15 pagesGeotech SHEAR BOX Final Report - MichaelMamattaMichael Adolf MamattaNo ratings yet
- Pacturan AEC51 Quiz 2Document2 pagesPacturan AEC51 Quiz 2CZARINA JANE ACHUMBRE PACTURANNo ratings yet
- Measuring Diffusivity of Carbon Tetrachloride Using TemperatureDocument19 pagesMeasuring Diffusivity of Carbon Tetrachloride Using TemperatureSohini RoyNo ratings yet
- Rock Mech 2019Document2 pagesRock Mech 2019Samarth ShuklaNo ratings yet
- UntitledDocument10 pagesUntitledMoe Oo HtunNo ratings yet
- Filtration. Oyola - Orozco - Caballero.Document9 pagesFiltration. Oyola - Orozco - Caballero.ALEXANDRA CABALLERO TURIZONo ratings yet
- Batch 6 Final ReviewDocument93 pagesBatch 6 Final Reviewlakkepogu satish kumarNo ratings yet
- T2 EosDocument17 pagesT2 EosKush ShahNo ratings yet
- Data SheetDocument2 pagesData SheetJussier VitorianoNo ratings yet
- Bakteri Growth Over TimeDocument3 pagesBakteri Growth Over TimeHikmahmutNo ratings yet
- PETE 311 Midterm Exam 2 Key Spring 2023 PDFDocument4 pagesPETE 311 Midterm Exam 2 Key Spring 2023 PDFDrake WellsNo ratings yet
- PHYS 194 Report2Document5 pagesPHYS 194 Report2AbdulNo ratings yet
- Soil Ex3Document5 pagesSoil Ex3Azeezan AlessaNo ratings yet
- UntitledDocument6 pagesUntitled김서현 / 학생 / 기계공학부No ratings yet
- Tugas 3 Kelompok 1 Dinamika Kapal BDocument3 pagesTugas 3 Kelompok 1 Dinamika Kapal BFirmansyah AuliaNo ratings yet
- Alkynyl-Functionalized Gold NHC Complexes and TheiDocument5 pagesAlkynyl-Functionalized Gold NHC Complexes and TheikarthickrajaNo ratings yet
- (IA) 2CsPb2Br7 SupportingDocument11 pages(IA) 2CsPb2Br7 SupportingNacho Delgado FerreiroNo ratings yet
- Investigating Size Reduction OkDocument13 pagesInvestigating Size Reduction OkRoselynNo ratings yet
- Matching Transformer Design Specification and Core Loss DataDocument3 pagesMatching Transformer Design Specification and Core Loss DataNaeemo IraqiNo ratings yet
- CL351: Chemical Engineering Lab-II Semester 1, 2014-2015 IIT GandhinagarDocument7 pagesCL351: Chemical Engineering Lab-II Semester 1, 2014-2015 IIT GandhinagarPradeep DiwakarNo ratings yet
- YA-Giordano-2003-viscosidad de Funddo Multicomponete No ArrenianoDocument15 pagesYA-Giordano-2003-viscosidad de Funddo Multicomponete No ArrenianoEdgar Iván Pérez Mendoza CBNo ratings yet
- Determination of Heat ConductionDocument10 pagesDetermination of Heat Conductionhanif yooNo ratings yet
- TTU Faculty of Engineering Torsion Test ReportDocument6 pagesTTU Faculty of Engineering Torsion Test ReportG. Dancer GhNo ratings yet
- End Semester Exam in Solid Fluid Mechanics (35mDocument3 pagesEnd Semester Exam in Solid Fluid Mechanics (35mDEEPAK KUMARNo ratings yet
- 28 TracerDiffusion PDFDocument11 pages28 TracerDiffusion PDFAkash KumarNo ratings yet
- EjercicosDocument8 pagesEjercicosdavidNo ratings yet
- Heat Transfer Lab Lab Report Experiment # 01: Study of Heat Conduction Through Copper BarDocument5 pagesHeat Transfer Lab Lab Report Experiment # 01: Study of Heat Conduction Through Copper BaryushiNo ratings yet
- - 22 - نسخةDocument10 pages- 22 - نسخةteaNo ratings yet
- Notches ProblemsDocument2 pagesNotches ProblemsRochakNo ratings yet
- Title: Particle Size Analysis Via Mechanical Sieve: CEE 346L - Geotechnical Engineering I LabDocument6 pagesTitle: Particle Size Analysis Via Mechanical Sieve: CEE 346L - Geotechnical Engineering I LabAbhishek RayNo ratings yet
- EDA ReportDocument10 pagesEDA ReportLeo Dominick MagpantayNo ratings yet
- Thapar University, Patiala Department of Chemical EngineeringDocument2 pagesThapar University, Patiala Department of Chemical EngineeringazsdxNo ratings yet
- Team 2 PresentationDocument10 pagesTeam 2 Presentationজোনাক বিহীন জীৱনNo ratings yet
- Vibrations SsDocument14 pagesVibrations SsOmar HaibaNo ratings yet
- Report#3 Testingthetensilestrengthofmetalsusingtheuniversaltestingmachine Chem1103l Group26Document8 pagesReport#3 Testingthetensilestrengthofmetalsusingtheuniversaltestingmachine Chem1103l Group26MarielleCaindecNo ratings yet
- Band Gap of CuODocument8 pagesBand Gap of CuOBilal JuttNo ratings yet
- Q5 Assignment MEC551Document7 pagesQ5 Assignment MEC551iqbal2609No ratings yet
- Direct Shear TestDocument10 pagesDirect Shear TestRuzengulalebih ZEta's-ListikNo ratings yet
- Cement Replace Wood AshDocument7 pagesCement Replace Wood AshM JOSEPH DAVID SELVANNo ratings yet
- Prractica 3 Termo 1 Steel Group 2,0Document11 pagesPrractica 3 Termo 1 Steel Group 2,0Brayan Chaupis GrimaldoNo ratings yet
- AnalysisDocument3 pagesAnalysisAnnamarie SanDiegoNo ratings yet
- Experiment 3 Batch (Differential Distillation) : 1. ObjectiveDocument13 pagesExperiment 3 Batch (Differential Distillation) : 1. ObjectiveDivyansh BhadauriaNo ratings yet
- Umbilical CalDocument1 pageUmbilical CalThejus PrakashNo ratings yet
- MOI - Sec 2 Su89-RRKDocument7 pagesMOI - Sec 2 Su89-RRKAryan ZutshiNo ratings yet
- Inductively Coupled Plasma-Mass Spectrometry: Practices and TechniquesFrom EverandInductively Coupled Plasma-Mass Spectrometry: Practices and TechniquesNo ratings yet
- Modular Forms and Special Cycles on Shimura Curves. (AM-161)From EverandModular Forms and Special Cycles on Shimura Curves. (AM-161)No ratings yet
- Earthquake Sample ExamDocument5 pagesEarthquake Sample ExamTipa LaoNo ratings yet
- Volcano CharadeDocument1 pageVolcano CharadeTipa LaoNo ratings yet
- CH 3 Stoichiometry Multiple ChoiceDocument6 pagesCH 3 Stoichiometry Multiple ChoiceSusie ZhangNo ratings yet
- Filipino Culture Manifest in Landscapes Rice As Staple Food Horror VacuiDocument1 pageFilipino Culture Manifest in Landscapes Rice As Staple Food Horror VacuiTipa LaoNo ratings yet
- Rizal LawDocument5 pagesRizal LawMathilda Victoria GomezNo ratings yet
- Sample Exam: Encircle The Letter of The Correct AnswerDocument5 pagesSample Exam: Encircle The Letter of The Correct AnswerTipa LaoNo ratings yet
- MSE 213 RRL ProposalDocument6 pagesMSE 213 RRL ProposalTipa LaoNo ratings yet
- MSE 225 Assessment PaperDocument1 pageMSE 225 Assessment PaperTipa LaoNo ratings yet
- MSE 225 Assessment PaperDocument1 pageMSE 225 Assessment PaperTipa LaoNo ratings yet
- Rizal LawDocument5 pagesRizal LawMathilda Victoria GomezNo ratings yet
- Sample ODocument2 pagesSample OTipa LaoNo ratings yet
- MatE 173 Library Work 8 - 25Document3 pagesMatE 173 Library Work 8 - 25Tipa LaoNo ratings yet
- Accomplishment ReportDocument2 pagesAccomplishment ReportTipa LaoNo ratings yet
- Session 22Document5 pagesSession 22Tipa LaoNo ratings yet
- Send To Ralph TonightDocument1 pageSend To Ralph TonightTipa LaoNo ratings yet
- Finding Nemo Reflection PaperDocument2 pagesFinding Nemo Reflection PaperTipa Lao0% (2)
- SomeDocument1 pageSomeTipa LaoNo ratings yet
- Accomplishment ReportDocument2 pagesAccomplishment ReportTipa LaoNo ratings yet
- Finding Nemo Reflection PaperDocument2 pagesFinding Nemo Reflection PaperTipa Lao0% (2)
- Peer work evaluation formDocument2 pagesPeer work evaluation formCarla May Cielo MacatangayNo ratings yet
- 1 s2.0 S0263876299718186 Main PDFDocument7 pages1 s2.0 S0263876299718186 Main PDFLeydi PatiñoNo ratings yet
- 14Document2 pages14Ananya GoelNo ratings yet
- Soalan Set1Document13 pagesSoalan Set1Aziz BakarNo ratings yet
- Thermoplastic Traffic PaintDocument132 pagesThermoplastic Traffic PaintEngr'Shemaiah JimenezNo ratings yet
- BS en 15345-2007Document16 pagesBS en 15345-2007Ali AnsiNo ratings yet
- Diffusivity of MEA in WaterDocument7 pagesDiffusivity of MEA in WaterHeng-Ji ChanNo ratings yet
- Merck: Safety Data SheetDocument6 pagesMerck: Safety Data SheetDiana Lucia MorantesNo ratings yet
- Control Valves Detail SheetDocument163 pagesControl Valves Detail SheetodivalentineNo ratings yet
- AP 2012 Physics B Free Response QuestionsDocument12 pagesAP 2012 Physics B Free Response QuestionsAlan G.No ratings yet
- Penjelasan Rumus FragstatDocument4 pagesPenjelasan Rumus FragstatSinom S. Probo HapsoroNo ratings yet
- Flexible Cellular Materials-Sponge or Expanded Rubber: Standard Specification ForDocument15 pagesFlexible Cellular Materials-Sponge or Expanded Rubber: Standard Specification Forari wiliamNo ratings yet
- Bulk Density of Fertilizer Loose ISO-3944-1980Document5 pagesBulk Density of Fertilizer Loose ISO-3944-1980fahim khattakNo ratings yet
- III 5 CropProcessing 1 8Document8 pagesIII 5 CropProcessing 1 8SannyBombeoJomocNo ratings yet
- Tubing Performance VLPDocument4 pagesTubing Performance VLPDhiaa LaMiNo ratings yet
- Dense Phase Co2 Transport PDFDocument87 pagesDense Phase Co2 Transport PDFSchöberl ErichNo ratings yet
- BS en 14316-1-2004Document30 pagesBS en 14316-1-2004محمد قديشيNo ratings yet
- Guerrero, Mary Justine A. - ChE 192 U - Heat Exchanger Design ProblemDocument15 pagesGuerrero, Mary Justine A. - ChE 192 U - Heat Exchanger Design ProblemJustine GuerreroNo ratings yet
- TRICOR TCM Coriolis Flow Meters - Range CatalogueDocument26 pagesTRICOR TCM Coriolis Flow Meters - Range CatalogueMedab Abd El MalekNo ratings yet
- The Density of Liquids and Solids & Viscosity of LiquidsDocument5 pagesThe Density of Liquids and Solids & Viscosity of LiquidsexobtsbapNo ratings yet
- Tutorial A Pipe System AnalysisDocument38 pagesTutorial A Pipe System AnalysisLuis OrtizNo ratings yet
- Rioflex - GX Series PDFDocument2 pagesRioflex - GX Series PDFosmar100% (1)
- Fluid Lecture NotesDocument90 pagesFluid Lecture Notessrutii100% (1)
- KNT-LT-1701 Lzt-1702abcDocument1 pageKNT-LT-1701 Lzt-1702abcdtt000001No ratings yet
- Chapter 5 - WEIGHT-VOLUME RELATIONSHIP PDFDocument44 pagesChapter 5 - WEIGHT-VOLUME RELATIONSHIP PDFKasturi Letchumanan100% (1)
- ASTM D 1439 - 97 Sodium CarboxymethylcelluloseDocument8 pagesASTM D 1439 - 97 Sodium Carboxymethylcellulosealin2005No ratings yet
- Asphaltene EclipseDocument16 pagesAsphaltene Eclipsenguyenhoangduc82No ratings yet
- ASTM C42M-16 Standard Test Method For Obtaining and Testing Drilled Cores and Sawed Beams of ConcreteDocument7 pagesASTM C42M-16 Standard Test Method For Obtaining and Testing Drilled Cores and Sawed Beams of ConcreteMalaz Abdul Jalil67% (3)
- Concretemixdesign 150329082925 Conversion Gate01Document167 pagesConcretemixdesign 150329082925 Conversion Gate01Saeed KhawamNo ratings yet
- CXC CXF CXI Flange CatalogDocument12 pagesCXC CXF CXI Flange CatalogPustinjak SaharicNo ratings yet
- EXPT 11 Intrinsic ViscosityDocument5 pagesEXPT 11 Intrinsic ViscosityBea A.100% (1)