Professional Documents
Culture Documents
Fluorescence: Fluorescence, Emission of Electromagnetic Radiation, Usually Visible Light, Caused by
Uploaded by
Frank Dotcom0 ratings0% found this document useful (0 votes)
10 views1 pageFluorescence
Original Title
Fluorescence
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentFluorescence
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
10 views1 pageFluorescence: Fluorescence, Emission of Electromagnetic Radiation, Usually Visible Light, Caused by
Uploaded by
Frank DotcomFluorescence
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 1
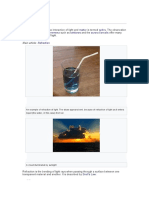
Fluorescence
Fluorescence, emission of electromagnetic radiation, usually visible light, caused by
excitation of atoms in a material, which then reemit almost immediately (within about
−8
10 seconds). The initial excitation is usually caused by absorption of energy from
incident radiation or particles, such as X-rays or electrons. Because reemission occurs so
quickly, the uorescence ceases as soon as the exciting source is removed, unlike
phosphorescence, which persists as an afterglow. A uorescent lightbulb is coated on the
inside with a powder and contains a gas; electricity causes the gas to emit ultraviolet
radiation, which then stimulates the tube coating to emit light. The pixels of a television or
computer screen uoresce when electrons from an electron gun strike them.
Fluorescence is often used to analyze molecules, and the addition of a uorescing agent
with emissions in the blue region of the spectrum to detergents causes fabrics to appear
whiter in sunlight. X-ray uorescence is used to analyze minerals.
This article was most recently revised and updated by William L. Hosch, Associate Editor.
You might also like
- Basics of Radiation and X-Rays in DentistryDocument32 pagesBasics of Radiation and X-Rays in DentistryM Ardi MaulanaNo ratings yet
- How X-Rays Work: Technology Was Invented Completely by Accident. in 1895, ADocument19 pagesHow X-Rays Work: Technology Was Invented Completely by Accident. in 1895, ASakhawat HussainNo ratings yet
- Bone LandmarkDocument3 pagesBone LandmarkDrexel DalaygonNo ratings yet
- The Study of Light and OpticsDocument3 pagesThe Study of Light and OpticsLeo CerenoNo ratings yet
- Fluorescence N PhosphorescenceDocument14 pagesFluorescence N Phosphorescenceanon_543130923No ratings yet
- Scattering and Absorption of LightDocument43 pagesScattering and Absorption of LightchalchisadeguNo ratings yet
- Photo Electric EffectDocument23 pagesPhoto Electric EffectAmarendra PandaNo ratings yet
- The Photoelectric EffectDocument2 pagesThe Photoelectric EffectJosh 'Cat' HigginsonNo ratings yet
- Light SourcesDocument2 pagesLight SourcesNinggen HoomanNo ratings yet
- LightDocument22 pagesLightangelicagagbo26No ratings yet
- Experiment 4: Fluorescent LightDocument3 pagesExperiment 4: Fluorescent Lightud54No ratings yet
- Notebook 16 PG 38-39Document2 pagesNotebook 16 PG 38-39api-334252501No ratings yet
- Radiation Sciences and TechnologyDocument43 pagesRadiation Sciences and TechnologyUmair AnsariNo ratings yet
- Fluorescence Under UV LightingDocument2 pagesFluorescence Under UV LightingRaluca AldeaNo ratings yet
- Band Gap Energy, Phosphorescence, and FluoroscenceDocument15 pagesBand Gap Energy, Phosphorescence, and FluoroscenceShaimah Rinda SariNo ratings yet
- Optical Properties PDFDocument27 pagesOptical Properties PDFaljhon100% (1)
- Assignment Analysis Final2Document8 pagesAssignment Analysis Final2Mahadi Hasan KhanNo ratings yet
- Theory of Light and ColorDocument50 pagesTheory of Light and Colorelijah jirah binadayNo ratings yet
- Photoelectric EffectDocument10 pagesPhotoelectric EffectSouvik BaruaNo ratings yet
- .Define X-Ray? Write Down The Properties of X-Ray?Document32 pages.Define X-Ray? Write Down The Properties of X-Ray?Sabbir HossainNo ratings yet
- FlourimetryDocument69 pagesFlourimetryAnonymous vr1l9Ylns100% (2)
- Fluorescence: Blanco Calderon Conde Padios Parales Sevilla TamagosDocument15 pagesFluorescence: Blanco Calderon Conde Padios Parales Sevilla TamagosHVEN .DNo ratings yet
- Laser: Vaneza Bea G. ABOGADODocument10 pagesLaser: Vaneza Bea G. ABOGADOVaneza Bea AbogadoNo ratings yet
- FLOURIMETRYDocument88 pagesFLOURIMETRYchannanjappamcNo ratings yet
- Lab Journal 2 Color Phenomena CompletedDocument8 pagesLab Journal 2 Color Phenomena CompletedMINH NGUYỄN LÂM TƯỜNGNo ratings yet
- 2007 Alexander Wunsch - Artifical Lighting and HealthDocument2 pages2007 Alexander Wunsch - Artifical Lighting and HealthalexandertaNo ratings yet
- Specular Diffuse:, or in Other Words, The Angle of Incidence Equals The Angle of ReflectionDocument4 pagesSpecular Diffuse:, or in Other Words, The Angle of Incidence Equals The Angle of ReflectionMaui ShihtzuNo ratings yet
- How Chlorophyll Absorbs and Emits LightDocument3 pagesHow Chlorophyll Absorbs and Emits LightabdulNo ratings yet
- What is Visible Light? Understanding the Spectrum of Light WavesDocument9 pagesWhat is Visible Light? Understanding the Spectrum of Light WavesJF BatucalNo ratings yet
- Photoelectric Effect:-: Electrons. This Phenomenon Is Commonly Studied in Electronic Physics, As Well AsDocument16 pagesPhotoelectric Effect:-: Electrons. This Phenomenon Is Commonly Studied in Electronic Physics, As Well AsSourav Paul100% (1)
- Photoelectric EffectDocument23 pagesPhotoelectric EffectkishoreNo ratings yet
- Opticalproperties (Page)Document22 pagesOpticalproperties (Page)Gift TanNo ratings yet
- Photon TheoryDocument20 pagesPhoton TheoryallanrnmanalotoNo ratings yet
- Phy 104 Atomic SpectraDocument24 pagesPhy 104 Atomic SpectraAisha abba HabibNo ratings yet
- Description: Electromagnetic Radiation Charged ParticlesDocument8 pagesDescription: Electromagnetic Radiation Charged ParticlesfaithNo ratings yet
- Photoelectric Effect1Document30 pagesPhotoelectric Effect1Valerie Anne AdoriasNo ratings yet
- Light Wave PropertiesDocument27 pagesLight Wave PropertiesDom Christian LastNo ratings yet
- Photochemistry-Ppt 7422144 PowerpointDocument10 pagesPhotochemistry-Ppt 7422144 PowerpointArangaNo ratings yet
- Photoelectric EffectDocument19 pagesPhotoelectric EffectSonu SinghalNo ratings yet
- Photochemistry: Dr. M.A Kazi Institute of Chemistry University of Sindh JamshoroDocument22 pagesPhotochemistry: Dr. M.A Kazi Institute of Chemistry University of Sindh JamshoroPeerBuxNo ratings yet
- Basic Mechanisms of Photoluminescence: 2.1 Excitation and Emission SpectraDocument20 pagesBasic Mechanisms of Photoluminescence: 2.1 Excitation and Emission SpectraSoumya BanerjeeNo ratings yet
- Physics Questions Asked ModernDocument4 pagesPhysics Questions Asked ModernAreesha soomroNo ratings yet
- Photoelectric Effect ExplainedDocument6 pagesPhotoelectric Effect ExplainedAyush PandeyNo ratings yet
- Photoelectric Effect ExplainedDocument14 pagesPhotoelectric Effect ExplainedTamilselvan BaskaranNo ratings yet
- Types of Light: Fluorescence, Triboluminescence, EtcDocument2 pagesTypes of Light: Fluorescence, Triboluminescence, EtcFelix HuNo ratings yet
- How The Ozone Layer WorksDocument2 pagesHow The Ozone Layer WorkscaridadNo ratings yet
- The Photoelectric Effect ExplainedDocument16 pagesThe Photoelectric Effect ExplainedRushita LingiahNo ratings yet
- 2- Interactions of Radiation With Matter (تم حفظه تلقائيا)Document39 pages2- Interactions of Radiation With Matter (تم حفظه تلقائيا)Bashar BassamNo ratings yet
- Radiation & Radioactivity: Department of Medical Biophysics and Informatics 3rd Medical Faculty of Charles UniversityDocument34 pagesRadiation & Radioactivity: Department of Medical Biophysics and Informatics 3rd Medical Faculty of Charles UniversityahkiaenaaaaNo ratings yet
- الإشعاع - WikipediaDocument80 pagesالإشعاع - WikipediaA.M.B M.B.M.ENo ratings yet
- Week 3 B Chapter 12 X-Ray Interaction With Matter 55Document37 pagesWeek 3 B Chapter 12 X-Ray Interaction With Matter 55Hanan AliNo ratings yet
- Physics ProjectDocument19 pagesPhysics Projectaneezabdul2003100% (1)
- Photoelectric Effect1Document11 pagesPhotoelectric Effect1Dêêpák Sîñgh ÑîtwálNo ratings yet
- Electromagnetic Waves & SpectrumDocument6 pagesElectromagnetic Waves & SpectrumAman LilaniNo ratings yet
- Joseph Von Fraunhofer Lines Spectroscopy Collimator Prism Lens InfiniteDocument6 pagesJoseph Von Fraunhofer Lines Spectroscopy Collimator Prism Lens InfiniteYousra OsmanNo ratings yet
- Light SeminarDocument9 pagesLight Seminarvarsham2No ratings yet
- Fotoelectric EffectDocument12 pagesFotoelectric EffectDavid IssaNo ratings yet
- Photoelectric Effect: Jump To Navigation Jump To SearchDocument10 pagesPhotoelectric Effect: Jump To Navigation Jump To SearchSrynnENo ratings yet
- Speakout Pronunciation Extra Elementary Unit 12Document1 pageSpeakout Pronunciation Extra Elementary Unit 12Frank DotcomNo ratings yet
- Electroplating - Britannica Online EncyclopediaDocument3 pagesElectroplating - Britannica Online EncyclopediaFrank DotcomNo ratings yet
- Speakout Pronunciation Extra Elementary Unit 9Document1 pageSpeakout Pronunciation Extra Elementary Unit 9Frank DotcomNo ratings yet
- Speakout Pronunciation Extra Elementary Unit 5Document1 pageSpeakout Pronunciation Extra Elementary Unit 5Frank DotcomNo ratings yet
- Speakout Pronunciation Extra Elementary Unit 7Document1 pageSpeakout Pronunciation Extra Elementary Unit 7Frank DotcomNo ratings yet
- Speakout Pronunciation Extra Elementary Unit 7Document1 pageSpeakout Pronunciation Extra Elementary Unit 7Frank DotcomNo ratings yet
- Speakout Pronunciation Extra Elementary Unit 8Document1 pageSpeakout Pronunciation Extra Elementary Unit 8Frank DotcomNo ratings yet
- Speakout Pronunciation Extra Elementary Unit 6Document1 pageSpeakout Pronunciation Extra Elementary Unit 6Frank DotcomNo ratings yet
- Speakout Grammar Extra Elementary Unit 3Document2 pagesSpeakout Grammar Extra Elementary Unit 3Angel David Arce Garcia0% (2)
- Speakout Pronunciation Extra Elementary Unit 1Document1 pageSpeakout Pronunciation Extra Elementary Unit 1Frank DotcomNo ratings yet
- Speakout Pronunciation Extra Elementary Unit 2 PDFDocument1 pageSpeakout Pronunciation Extra Elementary Unit 2 PDFFrank DotcomNo ratings yet
- Speakout Grammar Extra Elementary Unit 2Document2 pagesSpeakout Grammar Extra Elementary Unit 2Frank Dotcom100% (1)
- Speakout Grammar Extra Elementary Unit 4Document2 pagesSpeakout Grammar Extra Elementary Unit 4Catalina Garcia Donoso0% (1)
- Speakout Pronunciation Extra Elementary Unit 3Document1 pageSpeakout Pronunciation Extra Elementary Unit 3Frank Dotcom0% (1)
- Speakout Grammar Extra Elementary Unit 1Document2 pagesSpeakout Grammar Extra Elementary Unit 1Frank Dotcom100% (1)
- Ozone LayerDocument4 pagesOzone LayerFrank DotcomNo ratings yet
- Nuclear FusionDocument14 pagesNuclear FusionFrank DotcomNo ratings yet
- Cyanide Ion Selective Electrode User GuideDocument47 pagesCyanide Ion Selective Electrode User GuideFrank DotcomNo ratings yet
- Stokes's LawDocument1 pageStokes's LawFrank DotcomNo ratings yet
- Archimedes' PrincipleDocument2 pagesArchimedes' PrincipleFrank DotcomNo ratings yet
- Preparación de Curvas de CalibraciónDocument30 pagesPreparación de Curvas de Calibraciónjljimenez1969No ratings yet
- Differential EquationDocument2 pagesDifferential EquationFrank DotcomNo ratings yet
- MechanicsDocument69 pagesMechanicsFrank DotcomNo ratings yet
- Specific GravityDocument2 pagesSpecific GravityFrank DotcomNo ratings yet