Professional Documents
Culture Documents
Rile From NH4
Uploaded by
rrivera7396Original Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Rile From NH4
Uploaded by
rrivera7396Copyright:
Available Formats
Acetonitrile from NH4-acetate
Acetonitrile prep
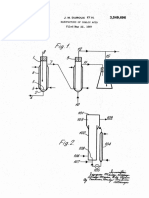
PATENT SPECIFICATION
Application Date: Oct 10, 1935 No 28007/35.
464,106 Complete Specification Left: Sept 23, 1936.
Complete Specification Accepted: April 12 ,1937 .
PROVISIONAL SPECIFICATION
Improvements in or relating to the Manufacture of Aliphatic Acid Nitriles We, BRITISH CFLANES:S LIMITED, a Company incorporated in accordance with the laws of Great Britain, of Celanese House, 22 & 23, Hanover Square, London, S W 1, HORACE FINNINGLEY OXLEY and EDWARD BOADEN THOMAS, both subjects of the King of Great Britain, of the Works of British Celanese Limited, Spondon , near Derby, do hereby declare the nature of this invention to be as follows:This invention relates to the manufacture of
aliphatie acid nitriles and especially acetonitrile and the nitriles of other lower fatty acids.
According to the invention an aliphatic acid nitrile is produced by decomposing by the action of heat the ammonium salt of the corresponding aliphatic acid in presence in the free state of a quantity of the same acid The production of acetonitrile, which is an important embodiment of the invention, is thus effected by decomposing amllonium acetate by the action of heat in presence of a quantity of free acetic acid.
In carrying out the process of the invention the ammonium salt to be decomposed may be heated under a fractionating column, together with a quantity of the corresponding acid in the free state, under conditions such that the nitrile will distil off as it is produced.
In such a process the presence of a quantity of free acid equal to about 40% of that combined in the ammonium salt gives excellent results and avoids the presence of substantial quantities of unchanged ammonium salt in the distillate.
Larger quantities of acid may be employed, if desired, although it is found that no advantage is secured by using a quantity of free acid greater than that combined in the salt.
4,5 In practice it has been found that the quantity of free acid thus employed may be reduced very substantially by carrying out the production of the nitrile as a continuous operation This object may be achieved by heating the mixture of ammonium salt and free acid to the decomposition temperature under a fractionating column, operated so as to permit distillation into a suitable receiver of the nitrile as it is formed, and provid 55 ing a continuous supply of fresh ammonium salt-acid mixture to the distillation vessel Operating in this manner it has been found possible to obtain excellent results by supplying to 60 the distillation vessel a mixture containing free acid equal to 15-20 % of that combined in the ammonium salt Such a mixture may be maintained in a liquid form suitable for continuous feeding by 65 warming e g to a temperature of 60-7 00 C.
In such a process the distillation vessel may, if desired, be charged initially with a mixture containing a rather larger 70 proportion of free acid than is present in the mixture continuously supplied; for instance the vessel may be charged with a mixture containing free acid in quantity equal to 20-50 % of the weight of 75 the acid combined in the ammonium salt employed.
The decomposition temperature employod may be varied according to the particular nitrile to be produced, and 80 according to other operating conditions.
In general, temperatures of 200-220 C.
are very suitable for the production of acetonitrile, temperatures of 200-210 C.
being very satisfactory Where, as will 85 usually be the case in practice, the distillation is effected under a fractionating column, the head of the column should be maintained at a temperature such that the nitrile distills over while the free 90 acid is as far as' possible returned to the distillation vessel In the production of acetonitrile a stillhead temperature of about 92 C has been found very satisfactory in practice 95 It has been found that the production of the nitriles can be accelerated very considerablv by the use of an iron catalyst.
The iron mav he introduced into the distillation vessel in the form of a compound 100 and phosphates of iron, e g Fe{ ( 12 POG) have been found very suitable for use in this manner Alternatively, a distillation vessel made of a corrosive-resistant ferrous alloy such a Stavbrite may be 105 employed, in which case it is found that % 00 %A, a ITT-246,O the small amount of corrosion which does take place is sufficient to provide the
iron catalyst.
Separation of the nitrile produced from the distillate obtained may be effected in any desired manner, for instance by fractional distillation, if desired, after the addition of sufficient acetic acid to convert all the acetate present into acid acetate and thus prevent distillation of ammonia.
The aqueous nitriles contaminated with the ammonium salt of the corresponding acid obtained in the process of the present invention may be purified very efficiently by a process comprising salting out the nitrile with the ammonium salt This may be effected by neutralising with ammonia free acid contained in the crude nitrile, and if necessary, adding a further quantity of the ammonium salt.
Such a salting out process produces in the case of acetonitrile an upper layer of aqueous nitrile of 95 % concentration, which, o-n fractionation, yields first a constant boiling nitrile-water mixture containing 84 % nitrile and subsequently the anhydrous nitrile It is possible by this means to obtain the bulk of the nitrile contained in the crude product in an anhydrous condition.
Fractionation of the ammonium acetate layer irom the salting out operation yields a distillate consisting of aqueous 35 nitrile containing free ammonia which can be used in neutralising a further batch of crude nitrile, and so likewise can ammonia obtained from the 84 % nitrile obtained by fractionating the 40 salted out product The residue from the fractionation of the ammonium acetate layer consists of ammonium acetate and the acid acetate and is available for use as starting material in the pro 45 duction of a further quantity of nitrile.
While the invention has been described above more particularly with regard to the manufacture of acetonitrile it is not limited in this respect and may be applied 50 to the manufacture of other aliphatic acid nitriles For example, by employing ammonium propionate as starting material propionitrile may be produced and likewise from ammonium butyrate 55 there may be obtained
butyronitrile .
Dated this 9th day of October, 1935.
STEPHENS & ALLEN, Chartered Patent Agents, Celanese House, 22 & 23, Hanover Square, London, W l.
COMPLETE SPECIFICATION
Improvements in or relating to the Manufacture of Aliphatic Acid Nitriles We, BRITISH CELANE Sn ,L Im I Tn D, a Company incorporated in accordance with the laws of Great Britain, of Celanese go House, 22 & 23, Hanover Square, London, W.1, HORACE FINNINGLEY OX:LY and E Dw ARD BOADEN T Hom As, both subjects of the King of Great Britain, of the Works of British Celanese Limited, d B Spondon , near Derby, do hereby declare the nature of this invention and in what manner the same is to be performed, to be particularly described and ascertained in and by the following statement:-
This invention relates to the manufacture of aliphatic acid nitriles and especially acetonitrile and the nitriles of other lower fatty acids.
According to the invention an aliphatic acid nitrile is produced by decomposing by the action of heat the ammonium salt of the corresponding aliphatic acid in presence in the free state of a quantity of the same acid The production of aceionitrile , which is an important embodiment of the invention, is thus effected by decomposing ammonium acetate by the action of heat in presence of a quantity of free acetic acid.
In carrying out the, process of the 85 invention the ammonium salt to be decomposed may be heated under a fractionating column, together with a quantity of the corresponding acid in the free state, under conditions such that the 90 nitrile will distil off as it is produced.
In such a process the presence of a quantity of free acid equal to about 40% of that combined in the ammonium salt gives excelent results and avoids the 95 presence of substantial quantities of unchanged ammonium salt in the distillate.
Larger quantities of acid may be employed, if desired, although it is found that no advantage is secured by 100 using a quantity of free acid greater than that combined in the salt.
In practice it has been found that the quantity of free acid thus employed may be reduced very substantially by carry 105 ing out the production of the nitrile as a continuous operation This object may be achieved by heating the mixture of ammonium salt and free acid to the decompqsition temperature under a frac 110 464,1 OU a 464,106 tion
ating column, operated so as to permit distillation into a suitable receiver of the nitrile as it is formed, and providing a continuous supply of fresh ammonium salt-
acid mixture to the distillation vessel Operating in this manner it has been found possible to obtain excellent results by supplying to the distillation vessel a mixture containing free acid equal to 15-90 % of that combined in the ammonium salt Such a mixture may be maintained in a liquid form suitable for continuous feeding by warming e g to a temperature of 60-700 C.
In such a process the distillation vessel may, if desired, be charged initially with a mixture containing a rather larger proportion of free acid than is present in the mixture continuously supplied; for instance the vessel may be charged with a mixture' containing free acid in quantity equal to 20-50 % of the weight of the acid combined in tbh ammonium salt employed.
The decomposition temperature employed may be varied according to the particular nitrile to be produced, and according to other operating conditions.
In general, temperatures of 200-2290 C.
are very suitable for the production of acetonitrile, temperatures of 200-210 C.
being very satisfactory Where, as will usually be the case in practice, the distillation is effected under a fractionating column, the head of the column should be maintained at a temperature such that the nitrile distills over while the free acid is as far as possible returned to the distillation vessel In the production of acetonitrile a still-head temperature of about, 920 C has been found very satisfactory in practice.
It has been found that the production of the nitriles can be accelerated very considerably by the use of an iron catalyst.
The iron may be introduced into the distillation vessel in the form of a compound and phosphates of iron, e g Fe( l 12 P 4), have been found very suitable for use in this manner Alternatively, a, distillation vessel made of a corrosive-resistant ferrous alloy such a
Staybrite may be employed, in which case it is found that the small amount of corrosion which does take place is sufficient to provide, the iron catalyst.
Separation of the nitrile produced from the distillate obtained may be effected in ( any desired manner, for instance by fractional distillation, if desired, after the addition of sufficient acetic acid to convert all the acetate present into acid acetate and thus prevent distillation of ammonia.
The aqueous nitriles conlandnated with the ammonium salt of the corresponding acid obtained in the process of the present invention may be purified very efficiently by a process comprising Malt 70 ing out the nitrile with the ammonium salt This may be efiected by neutralising with ammonia free acid contained in the crude nitrile, and if necessary adding a further quantity of the ammllo 75 nium salt.
Such a salting out process produces in the case of acetonitrile an upper layer of aqueous nitrile of 95 % concentration, which, on fractionation, yields first a con 80 stant boiling nitrile-water mixture containing 84 % nitrile and subsequently the anhydrous nitrile It is possible by this means to obtain the bulk of the nitrile contained in the crude product in an 85 anhydrous condition.
Fractionation of the ammonium acetate layer from the salting out operation yields a distillate consisting of aqueous nitrile containing free ammonia which 90 can be used in
neutralising a further batch of crude nitrile, and so likewise can ammonia obtained from the 84 % nitrile obtained by fractionating the salted out product The residue from 95 the fractionation of the ammonium acetate layer consists of ammonium acetate and the acid acetate and is available for use as starting material in the production of a further quantity of nitrile 100 While the invention has been described above more particularly with regard to the manufacture of acetonitrile it is not limited in this respect and may be applied to the manufacture of other aliphatic acid 105 nitriles For example, by employing ammonium propionate as starting material propionitrile may be produced and likewise from ammonium butvrate there may be obtained butyronitrile 110 The following Example illustrates the invention as applied to the production of acetonitrile:EXAMPLE .
A mixture of about 88 % amnionium 115 acetate and 12 % acetic acid, prepared by adding acetic acid to ammonium carbonate, is continuously fed to a still provided with a Viigreux column and containing either a quantity of the feed mixture 120 or a residue from a previous operation
You might also like
- HowToBeAnAss WhippingBoxer TextDocument160 pagesHowToBeAnAss WhippingBoxer Textrrivera7396100% (8)
- Treatise On Equity Jurisprudence Vol Iii - Pomeroy - 1918Document1,068 pagesTreatise On Equity Jurisprudence Vol Iii - Pomeroy - 1918rrivera7396100% (3)
- AlkylationDocument9 pagesAlkylationabhishek sharma100% (1)
- Stoic HandbookDocument24 pagesStoic HandbookRazvan Kxkxk100% (1)
- A Compendium of The Law of Property in Land by Edwards, William Douglas, 1848Document680 pagesA Compendium of The Law of Property in Land by Edwards, William Douglas, 1848rrivera7396No ratings yet
- EP000937029B1 Process for Ethyl Acetate Production from Ethanol OxidationDocument9 pagesEP000937029B1 Process for Ethyl Acetate Production from Ethanol OxidationMuhammad Yanuar AnantaNo ratings yet
- The Chemistry of Fertilisers and Manure - Including Information on the Chemical Constituents and Types of Fertilisers and ManuresFrom EverandThe Chemistry of Fertilisers and Manure - Including Information on the Chemical Constituents and Types of Fertilisers and ManuresRating: 5 out of 5 stars5/5 (1)
- Effortless by Greg McKeownDocument6 pagesEffortless by Greg McKeownNaison StanleyNo ratings yet
- Cp-117-Project EngineeringDocument67 pagesCp-117-Project Engineeringkattabomman100% (1)
- MANUFACTURING METHODS OF SULFURIC ACIDDocument11 pagesMANUFACTURING METHODS OF SULFURIC ACIDZamir Khan100% (3)
- Lesson Plan For DemoDocument7 pagesLesson Plan For DemoShiela Tecson GamayonNo ratings yet
- Process Steam Systems: A Practical Guide for Operators, Maintainers, and DesignersFrom EverandProcess Steam Systems: A Practical Guide for Operators, Maintainers, and DesignersNo ratings yet
- Integrated Process For The Production of Vinyl Acetate From Acetic Acid Via Ethyl AcetateDocument44 pagesIntegrated Process For The Production of Vinyl Acetate From Acetic Acid Via Ethyl Acetatearif ihwandaNo ratings yet
- Process for Manufacturing Acetic Acid Using Activated Copper Oxide CatalystDocument5 pagesProcess for Manufacturing Acetic Acid Using Activated Copper Oxide CatalystDAMIAN RAMOS CRISTHIAN JESUSNo ratings yet
- Atent Offitce: Patented July '5, 1927Document2 pagesAtent Offitce: Patented July '5, 1927Yustinus Selis ToronNo ratings yet
- Benzoic AcidDocument4 pagesBenzoic AcidAndy Wahyu HidayatNo ratings yet
- UNITED Starts: Patented Apr. 16, 1935Document2 pagesUNITED Starts: Patented Apr. 16, 1935shalsinia chantalNo ratings yet
- United States Patent O?ice: 15H: LLQ?ZDocument3 pagesUnited States Patent O?ice: 15H: LLQ?ZadamNo ratings yet
- Dec. 28, 1965 H. Roter Etal 3,226,188: Process For The Production of Aluminum Sulfate MeltDocument6 pagesDec. 28, 1965 H. Roter Etal 3,226,188: Process For The Production of Aluminum Sulfate MeltRia DevitasariNo ratings yet
- US3126422 EnglishDocument3 pagesUS3126422 EnglishMarike Bunga HarfintanaNo ratings yet
- US1960211 (Sudah)Document3 pagesUS1960211 (Sudah)aris_nurhidayatNo ratings yet
- Us2656248 PDFDocument4 pagesUs2656248 PDFchuckannabelleNo ratings yet
- AcetamideDocument4 pagesAcetamidejolouisNo ratings yet
- Jozmim: Feb. 17, 1953 B. V. Aller EtalDocument7 pagesJozmim: Feb. 17, 1953 B. V. Aller EtalFatonaRifkyPNo ratings yet
- Patent 01Document3 pagesPatent 01fatemeh afariNo ratings yet
- Continuous production of aluminum sulfateDocument4 pagesContinuous production of aluminum sulfateHector Andres CabezasNo ratings yet
- Efficient Process for Preparing Acetic Anhydride from AcetoneDocument3 pagesEfficient Process for Preparing Acetic Anhydride from AcetoneMary Grace VelitarioNo ratings yet
- United States Patent 0: '3, l50, l74 ICCDocument2 pagesUnited States Patent 0: '3, l50, l74 ICCMuhammadAmdadulHoqueNo ratings yet
- United States Patent: Patented June 15, 1971Document3 pagesUnited States Patent: Patented June 15, 1971cantikNo ratings yet
- United States: Patent OfficeDocument3 pagesUnited States: Patent OfficeIRIENE DELFITA TKIMNo ratings yet
- Filed June l5, 1935Document6 pagesFiled June l5, 1935Yustinus Selis ToronNo ratings yet
- United States Patent Office: As The Central Atom On A CarrierDocument4 pagesUnited States Patent Office: As The Central Atom On A CarrierRasoulNo ratings yet
- Process For AADocument15 pagesProcess For AASantiago BorgesNo ratings yet
- ,425,500. Patented Aug. 8, 1922.: H. W., Matheson and G, E, GrattanDocument3 pages,425,500. Patented Aug. 8, 1922.: H. W., Matheson and G, E, GrattanrzgarNo ratings yet
- Jan-27, 1959 ' - G. Baecklund 2,870,866: Method of Obtaining Acetaldehyde ' Filed June 14, 1952Document3 pagesJan-27, 1959 ' - G. Baecklund 2,870,866: Method of Obtaining Acetaldehyde ' Filed June 14, 1952MuhlisaApriliaNo ratings yet
- US4297290Process For Preparing Sorbitan EstersDocument5 pagesUS4297290Process For Preparing Sorbitan Esterstahera aqeelNo ratings yet
- US3639461Document5 pagesUS3639461M. IDRISNo ratings yet
- ' United States Patent Office : Ljatented Nov. 7, 195.0Document2 pages' United States Patent Office : Ljatented Nov. 7, 195.0Agape Ruth BaliloNo ratings yet
- US2373717Document2 pagesUS2373717Ruchita PoilkarNo ratings yet
- Catalytic Reduction of Carboxylic Acids to Aldehydes using Formic Acid under PressureDocument3 pagesCatalytic Reduction of Carboxylic Acids to Aldehydes using Formic Acid under Pressurebanjo010% (1)
- Indigo Prodn. From Phenyl-Glycine Carboxylic Acid Salt - by Fusion in Mixed Potassium Hydroxide and Sodium Hydroxide Melt, Then OxidnDocument4 pagesIndigo Prodn. From Phenyl-Glycine Carboxylic Acid Salt - by Fusion in Mixed Potassium Hydroxide and Sodium Hydroxide Melt, Then OxidnCillian CreedonNo ratings yet
- United States Patent (191: Inoue Et A) - (11) Patent Number: (45) Date of PatentDocument4 pagesUnited States Patent (191: Inoue Et A) - (11) Patent Number: (45) Date of PatentShrutiNo ratings yet
- Us Patent Manufacture of Urea, 1954Document4 pagesUs Patent Manufacture of Urea, 195425A Syifa Salsabila AlfianiNo ratings yet
- United States Patent Office: Patented Feb. 6, 1951Document3 pagesUnited States Patent Office: Patented Feb. 6, 1951karmilaNo ratings yet
- Arease: Nov. 8, 1966 W. Wogt Et Al 3,284,495 Process For The Continuous Manufacture, Purification andDocument3 pagesArease: Nov. 8, 1966 W. Wogt Et Al 3,284,495 Process For The Continuous Manufacture, Purification andRachmad HermawanNo ratings yet
- Us1951789 PDFDocument5 pagesUs1951789 PDFJames EdwardsNo ratings yet
- United States Patent Office: Patented June 29, 1948Document3 pagesUnited States Patent Office: Patented June 29, 1948jhartmann8No ratings yet
- Patented Process for Manufacturing UreaDocument2 pagesPatented Process for Manufacturing UreatreyzzztylerNo ratings yet
- Acetyl Salicylate PDFDocument3 pagesAcetyl Salicylate PDFtechkasambaNo ratings yet
- United States Patent 0: Patented Sept. 8, 1959Document4 pagesUnited States Patent 0: Patented Sept. 8, 1959olivia syifaNo ratings yet
- Producing Acetic Acid Anhydride Using Cobalt CatalystDocument2 pagesProducing Acetic Acid Anhydride Using Cobalt Catalystwilliam fathNo ratings yet
- Mm'mon FOR MAKIN: Filed Aug. 8, 1925Document4 pagesMm'mon FOR MAKIN: Filed Aug. 8, 1925arufatoNo ratings yet
- Chemical and Thermal Decomposition of Ammonium Sulphate Into Ammonia and Sulphuric AcidDocument3 pagesChemical and Thermal Decomposition of Ammonium Sulphate Into Ammonia and Sulphuric AcidRamona Mihaela VerdesNo ratings yet
- Office europeen des brevets (fi) Publication number : 0 4 8 2 753 A2Document16 pagesOffice europeen des brevets (fi) Publication number : 0 4 8 2 753 A2Lathifa Rahma AstutiNo ratings yet
- US PatentDocument4 pagesUS Patentaldo BMCNo ratings yet
- Us 3549696Document4 pagesUs 3549696budispartanNo ratings yet
- Patent Nines,: Nited StatesDocument2 pagesPatent Nines,: Nited Statesjeque661No ratings yet
- Aiiii: July 7, 1942. E. Mazabraud 2,289,286Document3 pagesAiiii: July 7, 1942. E. Mazabraud 2,289,286Özlem YılmazNo ratings yet
- US3093691Document2 pagesUS3093691Ayu GirlsNo ratings yet
- Logs:: ConcentratorDocument9 pagesLogs:: ConcentratorEniNo ratings yet
- Method For Producing Alkaline Salts of PhenylglycineDocument8 pagesMethod For Producing Alkaline Salts of PhenylglycineCillian CreedonNo ratings yet
- Equipment For Production of An Alkylation Plant For Igh-Octane GasolineDocument5 pagesEquipment For Production of An Alkylation Plant For Igh-Octane GasolinejoefrizalNo ratings yet
- Fig - F: March 30, 1954 M. J. Stu'RzmanDocument6 pagesFig - F: March 30, 1954 M. J. Stu'RzmanBernice JohnsonNo ratings yet
- Ethyl AcrylateDocument4 pagesEthyl AcrylateFirdaus ImamNo ratings yet
- Determination of Total Iron in Pharmaceutical PreparationsDocument3 pagesDetermination of Total Iron in Pharmaceutical PreparationsAnonymous droqJBqu6No ratings yet
- European Patent Application: Process and Plant For Ammonia-Urea ProductionDocument16 pagesEuropean Patent Application: Process and Plant For Ammonia-Urea ProductionRashminda AttanayakeNo ratings yet
- A Further Investigation of the Symmetrical Chloride of Paranitroorthosulphobenzoic AcidFrom EverandA Further Investigation of the Symmetrical Chloride of Paranitroorthosulphobenzoic AcidNo ratings yet
- The History of MacaDocument19 pagesThe History of Macarrivera7396No ratings yet
- John Ruiz's Boxing StrategyDocument5 pagesJohn Ruiz's Boxing Strategyrrivera7396No ratings yet
- jm060662k PDFDocument16 pagesjm060662k PDFrrivera7396No ratings yet
- Chapter 8 PresentationDocument26 pagesChapter 8 Presentationrrivera7396No ratings yet
- Triphala vs. ChlorhexidineDocument15 pagesTriphala vs. Chlorhexidinerrivera7396No ratings yet
- Massachusetts Criminal Procedure Rule 27 VerdictDocument2 pagesMassachusetts Criminal Procedure Rule 27 Verdictrrivera7396No ratings yet
- Never Break These Lab RulesDocument19 pagesNever Break These Lab Rulesrrivera7396No ratings yet
- Nutrition and You - Com Soybean Oil Nutrition FactsDocument5 pagesNutrition and You - Com Soybean Oil Nutrition Factsrrivera7396No ratings yet
- Knovenaegal KF and Al2O3Document4 pagesKnovenaegal KF and Al2O3rrivera7396No ratings yet
- SAMeDocument4 pagesSAMerrivera7396No ratings yet
- Esbit Hexamine MSDSDocument6 pagesEsbit Hexamine MSDSrrivera7396No ratings yet
- Rest IntervalsDocument3 pagesRest Intervalsrrivera7396No ratings yet
- Fiber Type and Exercise Intensity Chart for Rep Max TrainingDocument1 pageFiber Type and Exercise Intensity Chart for Rep Max Trainingrrivera7396No ratings yet
- Birches ExcerptDocument2 pagesBirches Excerptrrivera7396No ratings yet
- Microwave Friedel CraftsDocument5 pagesMicrowave Friedel Craftsrrivera7396No ratings yet
- 1 c4 b5Document2 pages1 c4 b5rrivera7396No ratings yet
- Alpha Bromination Benzyl CarbonDocument4 pagesAlpha Bromination Benzyl Carbonrrivera7396No ratings yet
- CeriumII) Chloride A Highly Efficient Reagent For The Synthesis of A-AminonitrilesDocument11 pagesCeriumII) Chloride A Highly Efficient Reagent For The Synthesis of A-Aminonitrilesrrivera7396No ratings yet
- Allyl Alcohol Interim Dec 2008 v1Document57 pagesAllyl Alcohol Interim Dec 2008 v1rrivera7396No ratings yet
- Hydroxylamine Aldehyde MicrowaveDocument4 pagesHydroxylamine Aldehyde Microwaverrivera7396No ratings yet
- Alchemical and Archaic Chemistry TermsDocument8 pagesAlchemical and Archaic Chemistry Termsrrivera7396No ratings yet
- Alchemical and Archaic Chemistry Terms: Part I (A-K)Document8 pagesAlchemical and Archaic Chemistry Terms: Part I (A-K)rrivera7396No ratings yet
- Cerium OxideDocument5 pagesCerium Oxiderrivera7396No ratings yet
- Big Bang TheoryDocument9 pagesBig Bang TheoryLoo DrBradNo ratings yet
- En GBDocument4 pagesEn GBahmedNo ratings yet
- LAC and Location UpdateDocument10 pagesLAC and Location UpdateAndres RockeNo ratings yet
- Shakuntala and Other Works, by KåalidåasaDocument255 pagesShakuntala and Other Works, by KåalidåasaMohamed Sayed AbdelrehimNo ratings yet
- Plumbing Layout and SpecificationsDocument1 pagePlumbing Layout and SpecificationsLiza P. PaculanangNo ratings yet
- Rebecca A. Endaya Beed-Iii Art Education: ExploreDocument5 pagesRebecca A. Endaya Beed-Iii Art Education: ExploreBhecca Endaya0% (1)
- HavellsDocument4 pagesHavellsanurag_iiitmNo ratings yet
- MARS Motor Cross Reference InformationDocument60 pagesMARS Motor Cross Reference InformationLee MausNo ratings yet
- MR - Abhishek JiDocument4 pagesMR - Abhishek Jimalikgaurav01No ratings yet
- Jurnal Aceh MedikaDocument10 pagesJurnal Aceh MedikaJessica SiraitNo ratings yet
- Comparing Means of Two GroupsDocument8 pagesComparing Means of Two GroupsRobert Kier Tanquerido TomaroNo ratings yet
- Vaiana Et Al (2021)Document11 pagesVaiana Et Al (2021)Raffaele CapuanoNo ratings yet
- Technical Data Sheet 01DT-1L..: Type OverviewDocument4 pagesTechnical Data Sheet 01DT-1L..: Type OverviewJNo ratings yet
- LUTS Spot TestDocument2 pagesLUTS Spot TestHardiTariqHamma100% (1)
- Literature Review BUS 507 PDFDocument18 pagesLiterature Review BUS 507 PDFtanmoy8554No ratings yet
- Datasheet PIC1650Document7 pagesDatasheet PIC1650Vinicius BaconNo ratings yet
- SYKES Home Equipment Agreement UpdatedDocument3 pagesSYKES Home Equipment Agreement UpdatedFritz PrejeanNo ratings yet
- Installation and Operating Instructions Gen-Key: Energy DivisionDocument22 pagesInstallation and Operating Instructions Gen-Key: Energy DivisionAnonymous RcxX0FcNo ratings yet
- Idioma IV Cycle Q1 Exam (2021-1) - STUDENTS ANSWERDocument9 pagesIdioma IV Cycle Q1 Exam (2021-1) - STUDENTS ANSWEREdward SlaterNo ratings yet
- Vision CSP22 Abhyaas Test 3SDocument44 pagesVision CSP22 Abhyaas Test 3SManasa DevarakondaNo ratings yet
- AA Practice Problems on Amino Acids and Peptides (less than 40 charsDocument20 pagesAA Practice Problems on Amino Acids and Peptides (less than 40 charsNurlaeli NaelulmunaMajdiyahNo ratings yet
- Alcatel 350 User Guide FeaturesDocument4 pagesAlcatel 350 User Guide FeaturesFilipe CardosoNo ratings yet
- RTL8185 Windows7 FixDocument2 pagesRTL8185 Windows7 FixJamesHackNo ratings yet
- Emg 1204 Introduction To Materials Science Tutorial I Attempt All These Questions Question OneDocument2 pagesEmg 1204 Introduction To Materials Science Tutorial I Attempt All These Questions Question Onesteve gateriNo ratings yet
- Configuring Cisco Easy VPN and Easy VPN Server Using SDM: Ipsec VpnsDocument56 pagesConfiguring Cisco Easy VPN and Easy VPN Server Using SDM: Ipsec VpnsrajkumarlodhNo ratings yet
- Neural Networks in Data Mining: Ripundeep Singh Gill, AshimaDocument6 pagesNeural Networks in Data Mining: Ripundeep Singh Gill, AshimaIOSRJEN : hard copy, certificates, Call for Papers 2013, publishing of journalNo ratings yet
- Microsoft PowerPoint Presentation IFRSDocument27 pagesMicrosoft PowerPoint Presentation IFRSSwati SharmaNo ratings yet