Professional Documents
Culture Documents
Theoretical Estimation of Viscosity of Oxygenates With Quinary Hydrocarbon Mixtures at 298.15 K
Uploaded by
Zet Van PersibOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Theoretical Estimation of Viscosity of Oxygenates With Quinary Hydrocarbon Mixtures at 298.15 K
Uploaded by
Zet Van PersibCopyright:
Available Formats
International Journal of Innovations in Biological and Chemical Sciences, Vol.
1: 16-21, 2011
16
THEORETICAL ESTIMATION OF VISCOSITY OF OXYGENATES WITH
QUINARY HYDROCARBON MIXTURES AT 298.15 K
Ranjan Dey
1
*, Prakash Chandra
2
, J. D. Pandey
2
1
Department of Chemistry, Birla Institute of Technology and Science (BITS),PILANI- K. K. BIRLA Goa
Campus, Zuarinagar 403726, Goa, India
2
Department of Chemistry, University of Allahabad, Allahabad- 211002, India
Abstract
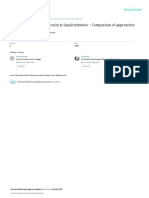
0.3
0.4
0.5
0 0.2 0.4 0.6 0.8 1
x
1
q
EXP
SUT
BING
K-M
ADD
On the basis of four different models, the theoretical estimation of viscosity of multicomponent liquid
mixtures has been carried out. The mixture under the present investigation is a regular pseudo binary
system, all the systems of an oxygenate (acetone, diisopropylether, methyl ethyl ketone, ethanol)
interacting with a five component hydrocarbon mixtures containing 25 mol % hexane, 10 mol % heptane, 35
mol % 2,2,4-trimethylpentane, 20 mol % toluene and 10 mol % p-xylene. The six component mixture has
been considered as a pseudo binary system. In order to carry out a comparative study, Kendall Munroe,
Bingham
and Additive equations have been employed. Furthermore, highlight of the work is the
computation of the viscosity by using the scarcely employed Sutherland relation in conjunction with
aforementioned viscosity models.
Key words : Viscosity, Hexanary, Multicomponent, Oxygenate
Introduction
The study of viscosity as a transport property is
being actively pursued by several workers because
of its significance in terms of design calculations
from the practical standpoint. It is not without
reason that multicomponent liquid systems have
attracted the attention of researchers in the past
few decades. Viscosity measurements of
multicomponent systems are found to exhibit
extremely significant multi dimensional industrial
applications such as in heat transfer, fluid flow,
mixing, agitation, heat exchangers and so forth.
Viscosity of photo resistant films play an important
role in maintaining the thickness and uniformity of
a coating layer on a wafer. Thus, information about
the viscosity of pure liquids and liquid mixtures is
very useful in several applications of chemical
technology. The estimation of viscosity of
multicomponent liquid mixture is much more
difficult than that of pure liquid.
From the theoretical aspect, it is equally
significant as it gives an in depth understanding of
*Corresponding author:
Email: drranjan@hotmail.com.
International Journal of Innovations in Biological and Chemical Sciences, Vol. 1: 16-21, 2011
17
the structure of liquid mixture. However, there is a
distinct scarcity of viscosity data of multicomponent
liquid mixtures; one may refer to some of the
papers
1-7
on the viscosity of ternary and quaternary
solutions. Thus, prediction or computation of
viscosities of multicomponent, in this case,
hexanary liquid mixtures at different temperatures,
is of paramount significance. This investigation
becomes more interesting as the methods
employed are simple yet deliver reasonably good
results.
Over the years, various empirical and semi-
empirical equations
8-10
for estimating liquid
viscosity have been proposed and developed. These
encompass Kendall-Munroe
8-10
, Frenkel
8-10
, Hind-
Ubbelhode
8-10
, Bingham
8-10
, Sutherland-Wassiljewa
8
and Additive
9
relations. Flory
10
theory and the
Bertrand- Acree- Burchfield
10
(BAB) approach have
also been employed to compute viscosity of
multicomponent liquid mixtures. Viscosity of
ternary, quaternary and quinary mixtures have also
been computed by making use of Pandey-
Prajapati
11
, Mason-Saxena
11
and the Lindsay-
Bromley
11
method. A number of models
12-14
for
predicting viscosity have been proposed including
Grunberg Nissan
12
, Katti-Chaudhry
12
, McAllister
12
,
Heric Brewer
12
, Eyring absolute rate theory
model
13
, corresponding-state theory
14,
significant
structure theory
14
, group contribution method
14
and model based on kinetic theory
14
. A critical
review of the viscosity data and their estimations
has been presented earlier
15
. A comprehensive
review of the entire empirical, semi empirical and
other methods employed for the estimation of
viscosity of binary liquid mixtures has been
presented by Lee and Lee
16
. Comparatively lesser
work has been done on the experimental and
theoretical aspects of viscosity of multicomponent
liquid systems. Extremely few studies are available
on the viscosity of multicomponent liquid mixtures
beyond ternary and quaternary due to the lack of
data. Asfour et al
17
measured accurately the density
and viscosity of a regular quinary system and its
quaternary subsystems at 298.15K. Recently
14
, the
analysis of viscosity data values of the
aforementioned quinary
17
and quaternary systems
was carried out by us.
The mixture under the present investigation is a
regular pseudo binary system, all the systems of an
oxygenate
18
(acetone, diisopropylether, methyl
ethyl ketone, ethanol) interacting with a five
component hydrocarbon mixtures containing 25
mol % hexane, 10 mol % heptane, 35 mol % 2,2,4-
trimethylpentane, 20 mol % toluene and 10 mol %
p-xylene. The six component mixture has been
considered as a pseudo binary system.
In order to carry out a comparative study,
Kendall Munroe
8
, Bingham
8
and Additive
8
equations have been employed. Furthermore,
highlight of the work is the computation of the
viscosity by using the scarcely employed
Sutherland
11
relation in conjunction with
aforementioned viscosity models. To the best of
our knowledge, such comparative data have not
been reported in literature so far.
Theoretical
A number of mathematical equations, based on
the viscosity data of pure components, have been
proposed by various workers for the computation
of viscosity of multicomponent systems.
In the present paper, Sutherland
equation
11
is applied to evaluate viscosity of
multicomponent liquid solutions. The aforesaid
equation can be expressed as,
1
1
i
m i ij
j
x
A
x
q q
( | |
= +
| (
\ .
. . . . (1)
where A
ij
is given by the expression
2
3 1
2 8
1
1
4
j
i
ij
j i
M
A
M
q
q
(
| | | |
( = +
| |
\ . \ . (
Here, A
ij
is the Wassiljewa coefficient
interpreted by Dey et al
8
and Pandey et al
11,14
as
the ratio of efficiencies with which molecules 'j' and
'i' impede the transport of momentum by
molecules 'i'. Sutherland, Wassiljewa and
Hirshfelder, using quite different approaches,
independently obtained Eq (1).
International Journal of Innovations in Biological and Chemical Sciences, Vol. 1: 16-21, 2011
18
Table 1 Viscosity and density values of the pure
components at 298.15 K
Component (g.cm
-3
) 10
-3
(Pa.s)
Ethanol 0.7849 1.0826
Acetone 0.7844 0.3029
Methyl ethyl ketone 0.7997 0.378
Diisopropylether 0.7185 0.319
2,2,4-Trimethylpentane 0.6877 0.472
n-Hexane 0.6548 0.307
n-Heptane 0.6795 0.3967
Toluene 0.8622 0.5525
p-Xylene 0.8565 0.605
Table 2 Calculated and experimental values of
viscosity of Acetone+Hydrocarbon mixture at
298.15K
x
1
exp SUT BING K-M ADD
10
-3
(Pa.s)
10
-3
(Pa.s)
10
-3
(Pa.s)
10
-3
(Pa.s)
10
-3
(Pa.s)
0.0000 0.404 - 0.450 0.437 0.431
0.0500 0.397 0.430 0.442 0.429 0.420
0.1001 0.391 0.424 0.435 0.421 0.409
0.2000 0.379 0.411 0.421 0.406 0.389
0.3000 0.368 0.398 0.406 0.392 0.372
0.4000 0.357 0.385 0.392 0.378 0.356
0.5000 0.347 0.372 0.377 0.365 0.343
0.6000 0.338 0.359 0.363 0.352 0.331
0.7000 0.329 0.345 0.348 0.340 0.321
0.7999 0.320 0.332 0.334 0.328 0.314
0.8498 0.316 0.325 0.327 0.322 0.311
0.8999 0.313 0.319 0.319 0.316 0.308
0.9500 0.309 0.312 0.312 0.311 0.306
1.0000 0.305 - 0.305 0.305 0.305
Table 3 Calculated and experimental values of
viscosity of Diisopropyl ether + Hydrocarbon
mixture at 298.15K
x
1
exp SUT BING K-M ADD
10
-3
(Pa.s)
10
-3
(Pa.s)
10
-3
(Pa.s)
10
-3
(Pa.s)
10
-3
(Pa.s)
0.0000 0.404 - 0.450 0.437 0.431
0.0500 0.399 0.432 0.443 0.430 0.421
0.1000 0.394 0.427 0.436 0.423 0.411
0.1999 0.384 0.417 0.423 0.410 0.394
0.3000 0.374 0.407 0.410 0.397 0.378
0.4000 0.364 0.397 0.397 0.384 0.364
0.5000 0.355 0.387 0.383 0.372 0.352
0.5999 0.346 0.377 0.370 0.360 0.341
0.6999 0.337 0.365 0.357 0.349 0.332
0.8000 0.328 0.352 0.344 0.338 0.325
0.8499 0.324 0.345 0.337 0.333 0.322
0.8999 0.320 0.337 0.330 0.327 0.320
0.9499 0.316 0.328 0.324 0.322 0.318
1.0000 0.312 - 0.317 0.317 0.317
Table 4 Calculated and experimental values of
viscosity of Methyl Ethyl ketone + Hydrocarbon
mixture at 298.15K
x
1
exp SUT BING K-M ADD
10
-3
(Pa.s)
10
-3
(Pa.s)
10
-3
(Pa.s)
10
-3
(Pa.s)
10
-3
(Pa.s)
0.0000 0.404 - 0.450 0.437 0.431
0.0500 0.399 0.435 0.446 0.433 0.424
0.1003 0.396 0.434 0.442 0.430 0.418
0.2003 0.391 0.432 0.435 0.424 0.407
0.3000 0.387 0.429 0.428 0.418 0.398
0.4000 0.384 0.425 0.421 0.412 0.389
0.5000 0.381 0.422 0.414 0.406 0.383
0.6000 0.379 0.417 0.407 0.400 0.378
0.7000 0.377 0.411 0.399 0.395 0.375
0.8000 0.375 0.403 0.392 0.389 0.373
0.8500 0.375 0.399 0.389 0.386 0.373
0.9000 0.375 0.393 0.385 0.383 0.374
0.9500 0.376 0.386 0.382 0.381 0.376
1.0000 0.377 - 0.378 0.378 0.378
Bingham
8
relation which takes into account
ideal mixing of liquids is given as
1
n
m i i
i
x q q
=
=
. . . . (2)
The viscosity of multicomponent systems,
according to the Kendall Munroe equation
8
, is
International Journal of Innovations in Biological and Chemical Sciences, Vol. 1: 16-21, 2011
19
given by
=
=
n
i
i i m
x
1
lnq q . . . . (3)
According to Additive relation
8
, viscosity is
given by
=
=
n
i
i i i m m
V x V
1
ln ln q q . . . (4)
where V is the molar volume.
Results and discussion
Viscosities of four, six component mixtures
(acetone, diisopropylether, methylethyl ketone and
ethanol with five component hydrocarbon
mixtures) at 298.15K, have been computed by
employing various empirical and semi-empirical
methods viz Sutherland, Bingham, Kendall
Munroe and Additive relations using Eqs (1), (2), (3)
& (4) respectively. The necessary data needed for
the computation and comparison have been taken
from literature
18
. All the parameters of pure
components are listed in Table-1. The systems
comprise of an oxygenate (acetone,
diisopropylether, methylethyl ketone, ethanol),
interacting with a five component hydrocarbon
mixture containing 25 mol % hexane, 10 mol %
heptane, 35 mol % 2,2,4-trimethylpentane, 20 mol
% toluene, and 10 mol % p-xylene. The six
component mixtures have been considered as
pseudo binary system consisting of a pure
component i.e. the oxygenate and another pseudo
pure component i.e. the hydrocarbon mixtures.
Tables 2, 3, 4 & 5 enlist the computed and
experimental values of viscosity of the pseudo
binary mixtures viz acetone + hydrocarbon mixture,
diisopropylether + hydrocarbon mixture, methyl
ethyl ketone + hydrocarbon mixture and ethanol +
hydrocarbon mixture respectively. Table 6 records
the average percentage deviations of the four
aforementioned pseudo binary mixtures computed
by the four different approaches at 298.15 K.
System-I: Acetone + hydrocarbon mixture:
From Table 2, it is clear that theoretically evaluated
viscosity values of pseudo binary mixture decrease
with increasing the mole fraction of the acetone.
Calculated values of viscosity show the same trend
as observed experimentally, and the maximum
percentage deviations are recorded for the
Bingham method and the minimum for the Additive
Table 5 Calculated and experimental values of
viscosity of Ethanol + Hydrocarbon mixture at
298.15K
x
1
exp SUT BING K-M ADD
10
-3
(Pa.s)
10
-3
(Pa.s)
10
-3
(Pa.s)
10
-3
(Pa.s)
10
-3
(Pa.s)
0.0000 0.404 - 0.450 0.437 0.431
0.0500 0.403 0.448 0.481 0.457 0.447
0.1000 0.406 0.460 0.513 0.478 0.465
0.2000 0.425 0.488 0.576 0.524 0.503
0.3001 0.446 0.521 0.640 0.574 0.545
0.4000 0.485 0.559 0.703 0.628 0.594
0.4999 0.532 0.605 0.767 0.688 0.649
0.6001 0.596 0.662 0.830 0.754 0.711
0.7000 0.671 0.731 0.894 0.825 0.783
0.8000 0.768 0.819 0.957 0.904 0.867
0.8500 0.829 0.872 0.989 0.946 0.915
0.8999 0.894 0.933 1.020 0.990 0.966
0.9499 0.977 1.003 1.052 1.036 1.022
1.0000 1.084 - 1.084 1.084 1.084
Table 6 Average percentage deviations by the different methods employed for the four oxygenates +
quinary hydrocarbon mixtures at 298.15 K.
Systems Average Percentage Deviation
Hydrocarbon
mixture +
Sutherland Bingham Kendall-Munroe Additive
Acetone 5.73 7.53 4.76 0.67
Diisopropyl Ether 7.68 7.03 4.67 1.21
Methyl Ethyl Ketone 8.5 7.21 5.48 1.82
Ethanol 10.34 27.97 19.06 15.54
International Journal of Innovations in Biological and Chemical Sciences, Vol. 1: 16-21, 2011
20
method. The APD values(Table 6) show the
following trend:
Additive< K-M< Sutherland< Bingham
System-II: Diisopropylether + hydrocarbon
mixture: A close observation of Table 3 indicates
that the viscosity values computed by four different
methods follow the same trend as observed
experimentally. For this pseudo binary system the
viscosity values show a gradually decreasing trend
with increasing mole fraction of oxygenate for all
the methods employed under the present
investigation. The APD values(Table 6) show the
following trend:
Additive< K-M< Bingham< Sutherland
System-III: Methyl ethyl ketone + hydrocarbon
mixture: A perusal of Table 4 indicates that the
viscosity values follow the general trend as
observed in the systems (I) and (II). For these
system, the viscosity values decrease with
increasing the mole fraction of first component i.e
methyl ethyl ketone. The APD values(Table 6) of the
pseudo binary system shows that the Additive
relation gives the best results while the deviations
are maximum in the case of Sutherland model.
Following trend is observed for this system:
Additive< K-M< Bingham< Sutherland
System-IV: Ethanol + hydrocarbon mixture: On
inspecting the results of Table 5, it has been
observed that for the pseudo binary system i.e
oxygenate with five hydrocarbon mixtures, the
viscosity values increase with increasing mole
fraction of ethanol. It is clear from the table that
computed values of viscosity are comparable and
found to be in good agreement with the
experimental findings. The APD values(Table 6) of
viscosity also show a very different trend as
compared to the other systems and are as follows:
Sutherland < Additive < K-M< Bingham
This discrepancy in the values may be attributed
to the presence of hydrogen bond between the
alcohol species. Some more factors responsible for
these values for the alkanol + alkane mixture may
be due to the hydrogen bond juncture and
dispersive interaction between unlike molecule
which might result in positive contribution to the
excess volume, dipole-dipole interaction and
geometrical fitting between components which
attribute to the negative contribution.
Conclusion
On the whole, a look at the APD values
point towards the fact that the values computed by
the additive approach are found to be best in terms
of agreement for systems(I), (II) and (III) under
consideration. Even, in system (IV), it is seen to give
the second best agreement. However, the
predictive methods employed are basically
empirical in nature and hence a completely
different trend might be observed for a different
set of systems. Overview of the results point out
the fact that all the four methods can be employed
quite successfully for the computation of viscosity,
since there seems to be reasonably good
agreement for all the methods employed in the
present investigation with exception to system(IV).
Thereby the discrepancies arising due to the
component present in the system are justified.
References
1. Fattahi, M and H. Iloukhani, 2010. Excess molar
volume, viscosity and refractive index study for
the ternary mixture { 2-methyl-2-butanol(1) +
tetrahydrofuran(2) + propylamine(3)} at
different temperatures. Application of the ERAS
model and Peng- Robinson- Stryjek- Vera
equation of state. Journal of Chemical
Thermodynamics, 42: 1335-1345.
2. Palani , R and A. Geetha, 2009. Acoustical and
excess thermodynamic studies of molecular
interactions in aqueous solvent systems at 303,
308 and 313 K. Physics and Chemistry of Liquids,
47(5) :542-552 .
3. Kao Yi-Chen and Chien - Hsuin Tu, 2011.
Densities, Viscosities, refractive indices and
surface tension for binary and ternary mixtures
of 2-propanol, tetrahydropyran and 2,2,4-
trimethlypentane . Journal of Chemical
Thermodynamics, 43: 218-256 .
4. Pandey, J.D., S. Haroon, J. Chhabra, R.Dey and K
Misra., 2005. Effect of Na
+
, K
+
and Ca
++
ions on
physicochemical properties of Thymine,
International Journal of Innovations in Biological and Chemical Sciences, Vol. 1: 16-21, 2011
21
Cytosine, Thymidine, and Cytidine in Urea
Solutions . Chinese Journal of Chemistry, 23:
1157-1164.
5. Johnson, I., M. Kalidoss and R. Srinivasmoorthy,
2002. Density, Viscosity and Speed of Sound in
the Ternary mixtures of 2-Ethoxyethanol+ N,N-
Dimethylformamide + N,N-Dimethylacetamide
and 2-Ethyxyethanol + Dimethyl Sulfoxide + N,N-
Dimethylacetamide at 308.15 K. Journal
Chemical Engineering Data, 47: 1388-1390.
6. Pandey, J D, V Vyas, P Jain, G.P. Dubey, N.
Tripathi and R Dey, 1999. Speed of sound,
viscosity & refractive index of multicomponent
system: Theoretical predictions from the
properties of pure components. Journal of
Molecular Liquids , 81 :123-133.
7. Shukla, R.K., S.K. Shukla, V.K. Pandey and Piyush
Awasthi, 2008. Excess internal pressure, excess
energy of vaporization and excess pseudo
Gruneisen parameter of binary, ternary and
quaternary liquid mixtures. 137: 104-109.
8. Dey, R., A. K. Singh and J. D. Pandey, 2008. A
Temperature dependent Viscometric Studies of
Binary Liquid Mixtures. Journal of Molecular
Liquids, 137:88-91.
9. Misra, A., I.Vibhu, R.K.Singh, M.Gupta and J.
Shukla, 2007. Acoustic , viscometric and optical
properties of binary mixtures of tetrahydrofuran
with 1-propanol and 2-propanol. Physics
Chemistry of Liquids, 45:93-104.
10. Pandey, J. D,. A. K Shukla, Ranjan Dey and V.
Sanguri, 1997 . Viscosity of multicomponent
liquid systems. Journal International Academy
of Physical Sciences, 1: 97-104.
11. Pandey, J. D., S. Mukherjee, M. K. Yadav and
Ranjan Dey, 2005. Viscosity of multicomponent
gas mixtures. Journal Indian Chemical Society,
82: 39-41
12. Ali, A., A.K. Nain, D.Chand and B Lal., 2007.
Ultrasonic, volumetric and viscometric studies of
molecular interactions in binary mixtures of
dimethylsulfoxide with polar substituted
cyclohexanes at 30C. Physics. Chemistry of
Liquids, 45:79-91,
13. Eyring, H, S. Glasstone and K. J. Laidler, 1941.
The theory of rate process, Mc Graw Hill Book
Co, New York.
14. Sanguri, V., N Singh, T.Srivastava, P.Chandra, N.
Pandey and J. D. Pandey, 2008.. Theoretical
estimation of viscosity of quinary and its
constituent quaternary liquid mixtures. Indian
Chem. Soc., 85: 948-925
15. Cao, W., A. Fredenslund and P. Rasmussen ,
1992. Statistical Thermodynamic Model for
viscosity for pure liquids and Liquid mixtures.
Industrial Engineering Chemical Research,
31: 2603-2619.
16. Lee, L. S., Y. S. Lee, 2001. The application of the
equation of state incorporated with mixing rules
for viscosity estimations for binary mixtures.
Fluid Phase Equilibria, 181 : 47-58.
17. Nhaesi, A. H. and A. F. Asfour, 2005. Densites
and viscosities of the regular quinary sytem:
Toleune(1) + Octane(2) + ethylbenzene(3) +
tetradecane(4) + hexadecane(5) and its
quaternary subsystems at 308.15 K and 313.15
K. Journal Chemical Engineering Data 50 :
149-153.
18. Peng, I. H. and C. H. Tu, 2002. Densities and
viscosities of acetone, Diisopropyl ether, ethanol
and methyl ethyl ketone with a five component
hydrocarbon mixture from 288.15 K to 308.15 K.
Journal Chemical Engineering Data, 47 : 1457-
1461.
You might also like
- Art RICCCE17 FedelesDocument8 pagesArt RICCCE17 FedelesAnca FedelesNo ratings yet
- The Determination of Viscosity at Liquid MixturesDocument7 pagesThe Determination of Viscosity at Liquid MixturesArtur FalcãoNo ratings yet
- Prediction of Activity Coefficients For Mixed Aqueous Electrolyte Solutions The Data of Their Binary SolutionsDocument11 pagesPrediction of Activity Coefficients For Mixed Aqueous Electrolyte Solutions The Data of Their Binary SolutionsWilo JaraNo ratings yet
- Volumetric, viscometric, spectral studies of ester-alcohol mixturesDocument20 pagesVolumetric, viscometric, spectral studies of ester-alcohol mixturesنجيب مفتاح المختار عمرNo ratings yet
- Costald 07-79Document11 pagesCostald 07-79boyd.george@bp.com100% (1)
- Tso NRTL 2ctu Tr444Document5 pagesTso NRTL 2ctu Tr444combo162No ratings yet
- Liquid-Liquid Equilibrium Data For Three Ternary Systems of Aqueous Alcohol Solutions AND Applicability of The Analytical Solutions of GroupsDocument3 pagesLiquid-Liquid Equilibrium Data For Three Ternary Systems of Aqueous Alcohol Solutions AND Applicability of The Analytical Solutions of GroupsGINA PAOLA BERRÍO JULIONo ratings yet
- Justel 2020. (NH4) 6Mo7O24 + Poly (Ethylene Glycol) + H2ODocument11 pagesJustel 2020. (NH4) 6Mo7O24 + Poly (Ethylene Glycol) + H2OYahaira Barrueto JhonsonNo ratings yet
- Alahmad 2010 - Prediction of Performance of Seawater RO UnitsDocument7 pagesAlahmad 2010 - Prediction of Performance of Seawater RO UnitsDánisa Urrutia ContrerasNo ratings yet
- Vis at Liq MixturesDocument6 pagesVis at Liq MixturesArian VelayatiNo ratings yet
- On The Applicability of The Uniquac Method To Ternary Liquid - Liquid EquilibriaDocument10 pagesOn The Applicability of The Uniquac Method To Ternary Liquid - Liquid EquilibriaHero19No ratings yet
- Fluid Phase EquilibriaDocument5 pagesFluid Phase EquilibriaMuzzy VoraNo ratings yet
- Art DIPE - 3Document6 pagesArt DIPE - 3Alex PintoiuNo ratings yet
- Unifac 6Document5 pagesUnifac 6lester33No ratings yet
- Morales - Jaime, 2019. Activity Coeff KClO4 + Poly (Ethylene Glycol) + H2ODocument10 pagesMorales - Jaime, 2019. Activity Coeff KClO4 + Poly (Ethylene Glycol) + H2OYahaira Barrueto JhonsonNo ratings yet
- Artículos para Introducción 3Document58 pagesArtículos para Introducción 3Carlos Mario Ortiz MuñozNo ratings yet
- Pc-Saft AssDocument6 pagesPc-Saft AssMartin FuenzalidaNo ratings yet
- Reactive DistillationDocument13 pagesReactive DistillationUtkarsh KapoorNo ratings yet
- Molecular Interactions of Surfactants With Polymer in Aqueous SolutionsDocument6 pagesMolecular Interactions of Surfactants With Polymer in Aqueous SolutionsDesi Rahma PrihandiniNo ratings yet
- Viscosity Coefficient and Activation Parameters For Viscous Flow of A Homologous Amino Acids in Aqueous Xylose SolutionsDocument11 pagesViscosity Coefficient and Activation Parameters For Viscous Flow of A Homologous Amino Acids in Aqueous Xylose SolutionstheijesNo ratings yet
- 3 - Isobaric - Vaporâ "Liquid - Equilibrium - For - Binary - System - of - Methanol - and - Acetonitrile PDFDocument4 pages3 - Isobaric - Vaporâ "Liquid - Equilibrium - For - Binary - System - of - Methanol - and - Acetonitrile PDFRogerNo ratings yet
- Methanol-water system interaction study reveals solvation behaviorDocument6 pagesMethanol-water system interaction study reveals solvation behaviorPablo Andrés Jiménez MamaniNo ratings yet
- LLE (Water + Butyric Acid +Document7 pagesLLE (Water + Butyric Acid +Endarto YudoNo ratings yet
- Partially Miscible LiquidsDocument8 pagesPartially Miscible LiquidsRenz Roger Esteves Buendicho100% (1)
- Correlation of Vapor-Liquid Equilibria Using Wilson Equation With Parameters Estimated From Solubility Parameters and Molar VolumesDocument11 pagesCorrelation of Vapor-Liquid Equilibria Using Wilson Equation With Parameters Estimated From Solubility Parameters and Molar VolumesLuciano GonçalvesNo ratings yet
- 276 2049 1 RVDocument7 pages276 2049 1 RVDrRamesh RedrouthuNo ratings yet
- Koneva 2017Document29 pagesKoneva 2017Novia Tri RamadaniNo ratings yet
- Ijert: On Prediction of Viscosity of Nanofluids For Low Volume Fractions of NanoparticlesDocument10 pagesIjert: On Prediction of Viscosity of Nanofluids For Low Volume Fractions of Nanoparticlesitsmeou11No ratings yet
- Estimation of Biodiesel Physical Propertiesusing Local Composition Based Models 2012 IEC Res 51 Pp13518Document9 pagesEstimation of Biodiesel Physical Propertiesusing Local Composition Based Models 2012 IEC Res 51 Pp13518Andreea Cristina PetcuNo ratings yet
- Estimation of Pure Component Properties. Part 4 - Estimation of The Saturated Liquid Viscosity of Non-Electrolyte Organic Compounds Via Group Contributions and Group InteractionsDocument23 pagesEstimation of Pure Component Properties. Part 4 - Estimation of The Saturated Liquid Viscosity of Non-Electrolyte Organic Compounds Via Group Contributions and Group InteractionscymyNo ratings yet
- Ternary Phase Diagrams For Aqueous Mixtures of Butyric Acid With Several Solvents: Experimental and Correlated DataDocument24 pagesTernary Phase Diagrams For Aqueous Mixtures of Butyric Acid With Several Solvents: Experimental and Correlated DataAbuALHASANNo ratings yet
- Phase Equilibria of Salt-Solvent-CO2 SystemsDocument9 pagesPhase Equilibria of Salt-Solvent-CO2 SystemsJuan Sebastian LopezNo ratings yet
- MM-ISMSA: An Ultrafast and Accurate Scoring Function For Protein Protein DockingDocument17 pagesMM-ISMSA: An Ultrafast and Accurate Scoring Function For Protein Protein Dockingharicoolguy111No ratings yet
- Isothermal Ciclohexane ELVDocument7 pagesIsothermal Ciclohexane ELValejzamora9No ratings yet
- Httpcpe Czasopisma Pan Plimagesdatacpewydaniano3201211calculationofvapourliquidliquidequilDocument15 pagesHttpcpe Czasopisma Pan Plimagesdatacpewydaniano3201211calculationofvapourliquidliquidequilLeonardoNo ratings yet
- 16 - 10 1016@j Fluid 2005 07 017Document12 pages16 - 10 1016@j Fluid 2005 07 017jasontodd22031995No ratings yet
- An Equation-Of-State-Based Viscosity Model For Non-Ideal Liquid MixturesDocument16 pagesAn Equation-Of-State-Based Viscosity Model For Non-Ideal Liquid MixturesDavid ReyesNo ratings yet
- 1.ISCA-RJCS-2012-001 DoneDocument12 pages1.ISCA-RJCS-2012-001 DoneChandra Icha KurniauanNo ratings yet
- Fluid Phase EquilibriaDocument8 pagesFluid Phase EquilibriagabogarreroNo ratings yet
- Temperature Dependence of Water Activity in Aqueous Solutions of SucroseDocument25 pagesTemperature Dependence of Water Activity in Aqueous Solutions of Sucrosepruebas123123100% (1)
- Experimental Liquid-Liquid EquilibriaDocument12 pagesExperimental Liquid-Liquid EquilibriaVadilsonMSantosNo ratings yet
- Leucine SolubilityDocument7 pagesLeucine SolubilityClarence AG YueNo ratings yet
- Determination of Intermolecular Interactions in Polar and Non-Polar Organic Molecules by Optical (Refractive Index) MethodDocument5 pagesDetermination of Intermolecular Interactions in Polar and Non-Polar Organic Molecules by Optical (Refractive Index) MethodchemistryjournalNo ratings yet
- Simulation of Reactive Distillation ColumnDocument6 pagesSimulation of Reactive Distillation ColumnthanhndbNo ratings yet
- AcetaminophenDocument9 pagesAcetaminophenRaziel456No ratings yet
- Rheology SucroseDocument6 pagesRheology SucroseCynthia LeBlancNo ratings yet
- Viscoelastic Properties and Orientation of Polymers in SolutionDocument3 pagesViscoelastic Properties and Orientation of Polymers in Solutionkishorkumarn8212No ratings yet
- Constants K and Alpha For Chitosan ViscometryDocument12 pagesConstants K and Alpha For Chitosan Viscometrydanny_ronaldNo ratings yet
- Study of Solid-Liquid Mixing in Agitated Tanks Through Computational Fluid Dynamics ModelingDocument10 pagesStudy of Solid-Liquid Mixing in Agitated Tanks Through Computational Fluid Dynamics ModelingrajuvadlakondaNo ratings yet
- Ijpap 2008Document7 pagesIjpap 2008btocarlNo ratings yet
- Group Contribution Method PDFDocument11 pagesGroup Contribution Method PDFVicente J Sandoval GNo ratings yet
- PhenolDocument7 pagesPhenolMartin FuenzalidaNo ratings yet
- Model and Optimisation of A Multi-Effect Evaporator of Sugarcane Juice: Energy Consumption and Inversion LossesDocument6 pagesModel and Optimisation of A Multi-Effect Evaporator of Sugarcane Juice: Energy Consumption and Inversion LossesakarczNo ratings yet
- General Quantitative Structure-Property Relationship Treatment of The Refractive Index of Organic CompoundsDocument5 pagesGeneral Quantitative Structure-Property Relationship Treatment of The Refractive Index of Organic CompoundsAlex-Mihai CiubaraNo ratings yet
- Predictive Group Contribution Models For The Thermophysical Properties of Ionic LiquidsDocument17 pagesPredictive Group Contribution Models For The Thermophysical Properties of Ionic LiquidsMohammad_Ismai_3096No ratings yet
- Fluid Phase Equilibria: Ling Li, Ting Zeng, Xiaoda Wang, Changshen Ye, Ting Qiu, Zhixian HuangDocument6 pagesFluid Phase Equilibria: Ling Li, Ting Zeng, Xiaoda Wang, Changshen Ye, Ting Qiu, Zhixian HuangvarunNo ratings yet
- Study of Hydrodynamics and Upscaling of Immiscible Fluid Stirred Tank Using Computational Fluid Dynamics SimulationDocument19 pagesStudy of Hydrodynamics and Upscaling of Immiscible Fluid Stirred Tank Using Computational Fluid Dynamics Simulationเอกฤกษ์ พุ่มนกNo ratings yet
- Modeling and Simulation of Azeotropic Distillation For Chloroform (1) + Methanol (2) + AcetoneDocument6 pagesModeling and Simulation of Azeotropic Distillation For Chloroform (1) + Methanol (2) + AcetoneAbhishek GadhwalNo ratings yet
- VLE Lactic Acid Ethyl Lactate Esterification PDFDocument7 pagesVLE Lactic Acid Ethyl Lactate Esterification PDFAseem Kashyap0% (1)
- Binary Polar Liquids: Structural and Dynamic Characterization Using Spectroscopic MethodsFrom EverandBinary Polar Liquids: Structural and Dynamic Characterization Using Spectroscopic MethodsRating: 5 out of 5 stars5/5 (1)
- Constant Force SpringDocument2 pagesConstant Force SpringSterlite DecorNo ratings yet
- Bus 172Document5 pagesBus 172api-538674995No ratings yet
- Mercury Pollution A Threat To Agusan MarshDocument25 pagesMercury Pollution A Threat To Agusan MarshKalambuan Foundation, Inc.50% (4)
- Chapter 3Document6 pagesChapter 3Sheldon BazingaNo ratings yet
- Tutorial 3 AnswersDocument2 pagesTutorial 3 AnswersM Arif SiddiquiNo ratings yet
- Presentation NishantDocument42 pagesPresentation NishantNishant PandaNo ratings yet
- Basics of Robotics KTUDocument6 pagesBasics of Robotics KTUBoni SamuelNo ratings yet
- Tutorial 1Document2 pagesTutorial 1eddy50% (2)
- Titanium Book From Org PDFDocument45 pagesTitanium Book From Org PDFSuthirak SumranNo ratings yet
- Machine Learning Notes - Lec 03 - Gender Identification Using Scikit-LearnDocument71 pagesMachine Learning Notes - Lec 03 - Gender Identification Using Scikit-LearnZara JamshaidNo ratings yet
- Bentley RM Bridge Advanced Detalii Module AditionaleDocument4 pagesBentley RM Bridge Advanced Detalii Module AditionalephanoanhgtvtNo ratings yet
- 01 AutoPIPE Vessel Fundamentals Introduction PPTDocument10 pages01 AutoPIPE Vessel Fundamentals Introduction PPTInamullah KhanNo ratings yet
- Calculate Warp and Weft For Balanced Fabric: Length of One Warp Thread Calculate Warp WidthDocument6 pagesCalculate Warp and Weft For Balanced Fabric: Length of One Warp Thread Calculate Warp WidthMajid KhanNo ratings yet
- Structure Design Calculation SheetDocument5 pagesStructure Design Calculation SheetifyNo ratings yet
- Why Python For GISDocument3 pagesWhy Python For GISalebeley21No ratings yet
- Color TheorypdfDocument99 pagesColor TheorypdfNews OffbeatNo ratings yet
- Engineering Drawing ScalesDocument18 pagesEngineering Drawing ScalesShadabNo ratings yet
- Part A1 Chapter 2 - ASME Code Calculations Stayed Surfaces Safety Valves FurnacesDocument25 pagesPart A1 Chapter 2 - ASME Code Calculations Stayed Surfaces Safety Valves Furnacesfujiman35100% (1)
- 4th SemesterDocument2 pages4th SemesterAbdul Rehman ButtsarkarNo ratings yet
- Powerful Presentation Tool BeamerDocument85 pagesPowerful Presentation Tool BeamerOtmane El ouardiNo ratings yet
- Slot18 2D Graphics ImagesDocument23 pagesSlot18 2D Graphics ImagesHuy KunnNo ratings yet
- SQL Server Versions in Distribution, Parallelism and Big Data - Paper - 2016Document9 pagesSQL Server Versions in Distribution, Parallelism and Big Data - Paper - 2016ngo thanh hungNo ratings yet
- Entry Test Preparation Test No.2 (ECAT)Document3 pagesEntry Test Preparation Test No.2 (ECAT)NAEEM UR REHMANNo ratings yet
- Question Bank AAIDocument4 pagesQuestion Bank AAIANANYA VNo ratings yet
- Filtration EquipmentsDocument29 pagesFiltration EquipmentsharijayaramNo ratings yet
- Internship Report (HR)Document47 pagesInternship Report (HR)Don’t KnowNo ratings yet
- PPTDocument19 pagesPPTSumit Kashyap100% (1)
- Preparation of Sample For AnalysisDocument27 pagesPreparation of Sample For Analysisapi-26215965100% (2)
- Wang Invited Proc.7195 PhotonicsWest09 Vytran 2009Document11 pagesWang Invited Proc.7195 PhotonicsWest09 Vytran 2009kndprasad01No ratings yet
- 4th Quarter Long Quiz in ScienceDocument2 pages4th Quarter Long Quiz in ScienceEderlina Bentilanon FagtananNo ratings yet