Professional Documents
Culture Documents
BaseCatalyzedOxidation ReductionofAldehydesbytheCannizzaroReaction
Uploaded by
Mike TranOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
BaseCatalyzedOxidation ReductionofAldehydesbytheCannizzaroReaction
Uploaded by
Mike TranCopyright:
Available Formats
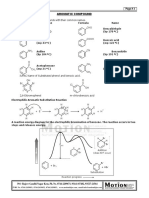
Base-Catalyzed Oxidation-Reduction of Aldehydes by the Cannizzaro Reaction
Prepared by Mike Tran THEORY The purpose of this experiment is to oxidize and reduce an aromatic aldehyde, 4-chlorobenzaldehyde, and isolated to form benzoic acid and benzyl alcohol. MAIN REACTION(S) AND MECHANISMS The Cannizzaro reaction consists of an oxidation-reduction reaction between a hydroxide ion and aldehydes without -hydrogen atoms.
With the presence of a strong base, the first aldehyde molecule reduces to a second aldehyde molecule and then becomes a primary alcohol. Within this process the molecule oxidizes itself to the carboxylate anion and will result in a carboxylic acid and an alcohol.
PROCESS The course of this experiment was broken up into multiple scenarios: setting up and preparing the reagents for the reaction, the reaction itself, and then the isolation and purification process of the products. To prepare the reagents, potassium hydroxide (5.0 g) was dissolved in distilled water (5 mL) inside an Erlenmeyer flask. 4chlorobenzaldehyde (1.0 g) and methanol (2.5 mL) was added to to a 25 mL round bottom flask with a stir bar and set to dissolve as well. An
apparatus was also set up for heating under reflux and simple distillation. Then, potassium hydroxide (50% aqueous, 1.5 mL) was added to the round bottom flask reaction vessel and set to heat under reflux so that none of the reactants were able to escape during the experiment. A water bath was prepared so that temperature was more easily regulated rather than using sand. This temperature was set to 75 degrees C and the reaction vessel underwent the heating process for 1.5 hours with automatic stirring from the magnetic stir bar. Once the heating under reflux stage was complete, the solution was transferred to a separatory funnel for separation of the two products. Although both solutions were transparent, it was easy to distinguish that the reaction formed two layers of compounds. The lower layer was the organic layer and the upper was the aqueous layer. Each layer was then extracted to separate beakers as the Cannizzaro reaction was not complete. The carboxylic acid was in the aqueous layer and the alcohol was in the methylene chloride (organic) layer. To recover the p-chlorobenzyl alcohol, the methylene chloride layer was wash it with two portions saturated aqueous sodium chloride (2x5 mL) as this will neutralize the solution of residual acid. Once the layer has been extracted, it was dried with several spatula tips of anhydrous sodium sulfate until the solution turned clear. The solution underwent simple distillation to remove the dichloromethane, resulting in 4chlorobenzyl alcohol leftover. To recover the p-chlorobenzoic acid, it was cooled by placement inside an ice-water bath and acidified by adding concentrated hydrochloric acid (2.5 mL) until it was acidic (approximately pH = 3). It was then filtrated with a Hirsh funnel and allowed for collection of the white precipitate product, p-chlorobenzoic acid. This was set out to dry prior to gathering the products weight and melting point.
DATA AND OBSERVATIONS After the chemical components were added to recover the acid and the alcohol layer, I had completely mixed up which was the organic layer and which was the aqueous layer. Hydrochloric acid was added to the
alcohol layer so to make the solution acidic. Litmus paper was used periodically to check the acidity of the solution to make sure it was approximately at the pH level of 3. The acid layer had 5 mL of anhydrous sodium sulfates and 2.5mL of hydrochloric acid added to it before the product was filtrated out of the solution. The product of the alcohol layer was a cloudy white color when extracted and yielded white crystals when weight and melting point were measured.
RESULTS
Product Data for Alcohol Layer Theoretical Actual % Yield Yield Yield 4.03 g 1.25 g 31%
Product p-chlorobenzyl alcohol
Actual m.p 68-69
Literature m.p 70-72
Product p-chlorobenzoic acid
Product Data for Acid Layer Theoretical Actual % Yield Yield Yield 0.215 g 0.11 g 51%
Actual m.p 166-172
Literature m.p 149-150
Percent Yield Aqueous Layer (0.09g / 0.289g) x 100 = 31% recovered Organic Layer (0.1464g / 0.285g) x 100 = 51% recovered CONCLUSION The p-chlorobenzyl alcohol from the alcohol layer was pure within a 1 degree C melting point range and a 3.5% error, however only 31% of the product was retrieved. Such a low percent yield could have been caused by the acid and the other chemicals added to acidify the solution after mistaking the two different layers. The results found with the p-chlorobenzoic acid from the acid layer however did not show
such promising results. The melting point range was 6 degree C, which indicates that the product was not pure and something went wrong in the experiment. Again, this and the 51% product retrieved may be explained by the mix up of the two different layers.
You might also like
- Chem 31.1 Experiment 9 Synthesis of Organic CompoundsDocument68 pagesChem 31.1 Experiment 9 Synthesis of Organic Compoundshello87623100% (1)
- Exp 1Document5 pagesExp 1Nur AthirahNo ratings yet
- Experiment 11Document8 pagesExperiment 11ShennyKoh100% (8)
- Preparation of 4-MethylcyclohexeneDocument5 pagesPreparation of 4-Methylcyclohexenemh1361410% (1)
- Experiment 9Document6 pagesExperiment 9clairedemotica100% (1)
- Experiment: 3 Extraction Technique: Liquid-Liquid ExtractionDocument7 pagesExperiment: 3 Extraction Technique: Liquid-Liquid ExtractionNatasha ClementNo ratings yet
- Lab 2 - Extraction and RecrystallizationDocument4 pagesLab 2 - Extraction and RecrystallizationJoshua Smith100% (2)
- Qualitative tests for plant compoundsDocument9 pagesQualitative tests for plant compoundsvishwanathz47No ratings yet
- Experimen 5 Organic ChemistryDocument8 pagesExperimen 5 Organic ChemistryAbd RaHmanNo ratings yet
- 18551507-019 CHEM-312 Lab ManualDocument8 pages18551507-019 CHEM-312 Lab ManualRukhsana Malik100% (1)
- Hydrolysis of Methyl Salicylate ExpDocument7 pagesHydrolysis of Methyl Salicylate ExpPradeep100% (1)
- Chemistry 125 Laboratory 11Document5 pagesChemistry 125 Laboratory 11SmaeUBNo ratings yet
- Extraction of Alkene From AlcoholDocument4 pagesExtraction of Alkene From AlcoholMsShu93No ratings yet
- Organic Chemistry Laboratory I BSK1402 Lab ReportDocument10 pagesOrganic Chemistry Laboratory I BSK1402 Lab ReportCucu AlbertNo ratings yet
- exp 2Document5 pagesexp 2zanjinyadzaNo ratings yet
- Lab 20 Synthesis of Banana OilDocument7 pagesLab 20 Synthesis of Banana OilgioNo ratings yet
- 1 s2.0 S0021925818767616 MainDocument8 pages1 s2.0 S0021925818767616 Mainaviral151402No ratings yet
- Chem Lab Report 2 Deol ADocument5 pagesChem Lab Report 2 Deol AMagnolia Kaye DeolaNo ratings yet
- Experiment 9Document6 pagesExperiment 9Anonymous s4HW3TX0IHNo ratings yet
- Cannizaro ReactionDocument13 pagesCannizaro Reactionhussein alnasryNo ratings yet
- CyclohexeneDocument13 pagesCyclohexeneRana BlackNo ratings yet
- Exp 3-Reduction of Cyclohexanone With Sodium BorohydrideDocument11 pagesExp 3-Reduction of Cyclohexanone With Sodium Borohydrideakuserai100% (3)
- Lab 3 Aspirin ReportDocument3 pagesLab 3 Aspirin ReportMsShu93100% (1)
- Experiment 53: Acetylation of GlucoseDocument3 pagesExperiment 53: Acetylation of GlucoseGeena John100% (1)
- 2C-B Synthesis Without LAH PDFDocument4 pages2C-B Synthesis Without LAH PDFatomosco100% (3)
- Lab Report 1Document10 pagesLab Report 1sheril nur hazianiNo ratings yet
- Synthesize Alkyl HalideDocument6 pagesSynthesize Alkyl HalideAnna Sophia EbuenNo ratings yet
- CyclohexeneDocument11 pagesCyclohexeneanon-407590100% (10)
- KOH synthetic routesDocument6 pagesKOH synthetic routesCin D NgNo ratings yet
- Formal Report, Carboxylic Acid and DerivativesDocument4 pagesFormal Report, Carboxylic Acid and DerivativesVicente Romeo M Macatangay88% (8)
- Derivative Vitamin C Application Synthesis of Labelled Ascorbic AcidDocument3 pagesDerivative Vitamin C Application Synthesis of Labelled Ascorbic Acidزياد الحسناويNo ratings yet
- Organic Chemistry Lab: Synthesis of Octyl AcetateDocument6 pagesOrganic Chemistry Lab: Synthesis of Octyl AcetateKristine BautistaNo ratings yet
- CE - Experiment 2 Extraction With Acid and AlkalineDocument8 pagesCE - Experiment 2 Extraction With Acid and AlkalineWeiChingNo ratings yet
- Organic Chemistry Practical 301Document22 pagesOrganic Chemistry Practical 301geetesh waghela100% (1)
- Experiment 8 Preparation of Cyclohexene From CyclohexanolDocument6 pagesExperiment 8 Preparation of Cyclohexene From CyclohexanolAishah Cnd100% (1)
- Optimized 2C-B Synthesis via Nitrostyrene ReductionDocument1 pageOptimized 2C-B Synthesis via Nitrostyrene ReductionFermin GamboaNo ratings yet
- CHEM F110 - Lab Manual - Nov 5-2020Document45 pagesCHEM F110 - Lab Manual - Nov 5-2020STUTI MATHUR100% (2)
- CHM 457 Lab 1Document7 pagesCHM 457 Lab 1suraini100% (1)
- Experiment 9 - : Alkene Synthesis From Alcohol Preparation of Cyclohexene From CyclohexanolDocument6 pagesExperiment 9 - : Alkene Synthesis From Alcohol Preparation of Cyclohexene From CyclohexanolSoo Hui Yan0% (2)
- Isolation of Eugenol from ClovesDocument8 pagesIsolation of Eugenol from ClovesAngeliqueNo ratings yet
- CH 26 AA - Montano - Jiara - SN and E ReactionsDocument3 pagesCH 26 AA - Montano - Jiara - SN and E ReactionsJiara MontañoNo ratings yet
- TheoryDocument3 pagesTheorymnukwa wendieNo ratings yet
- Synthesis of An Alkyl HalideDocument4 pagesSynthesis of An Alkyl HalideClyde Co SorianoNo ratings yet
- Fractional Distillation XXXXDocument3 pagesFractional Distillation XXXXMikee MeladNo ratings yet
- DiazepamDocument7 pagesDiazepamjiskate77No ratings yet
- Chem - Expt 10Document4 pagesChem - Expt 10Mirzi TurbolenciaNo ratings yet
- Grade Xii Practical ContentDocument7 pagesGrade Xii Practical ContentAvi ANo ratings yet
- Tests For CarbohydratesDocument19 pagesTests For CarbohydratesKenneth CatacutanNo ratings yet
- Experiment 9 Organic Chemistry LabDocument7 pagesExperiment 9 Organic Chemistry LabRhodelyn TolentinoNo ratings yet
- Classify Carboxylic Acids and Derivatives TestsDocument2 pagesClassify Carboxylic Acids and Derivatives TestsCarla PulgarNo ratings yet
- Deamination Lab ReportDocument4 pagesDeamination Lab ReportRyanJForteNo ratings yet
- Synthesis of Tert-Butyl Chloride from AlcoholDocument7 pagesSynthesis of Tert-Butyl Chloride from AlcoholFerdinand Tamayo Cayabyab Jr.No ratings yet
- Recrystallization and Manufacture of Aspirin: The Practicum of Organic ChemistryDocument21 pagesRecrystallization and Manufacture of Aspirin: The Practicum of Organic ChemistryEra MelaniaNo ratings yet
- GEMs ID90Document8 pagesGEMs ID90Andre PNo ratings yet
- Synthesize and Evaluate CinnamaldehydeDocument26 pagesSynthesize and Evaluate CinnamaldehydeArra Maeva Gacusana0% (1)
- PAC 213 prac 1Document6 pagesPAC 213 prac 1Tlotliso MphomelaNo ratings yet
- Advanced Pharmaceutical analysisFrom EverandAdvanced Pharmaceutical analysisRating: 4.5 out of 5 stars4.5/5 (2)
- The Chemistry of Fertilisers and Manure - Including Information on the Chemical Constituents and Types of Fertilisers and ManuresFrom EverandThe Chemistry of Fertilisers and Manure - Including Information on the Chemical Constituents and Types of Fertilisers and ManuresRating: 5 out of 5 stars5/5 (1)
- The Chemistry of Dairy Products - A Chemical Analysis of Milk, Cream and ButterFrom EverandThe Chemistry of Dairy Products - A Chemical Analysis of Milk, Cream and ButterNo ratings yet
- Aldehyde, Ketone Jee 5 Years MCQDocument162 pagesAldehyde, Ketone Jee 5 Years MCQbhagirathNo ratings yet
- Soap Manufacturing Process GuideDocument4 pagesSoap Manufacturing Process GuideNayan Gautam100% (1)
- June 2002 Question Paper 1Document20 pagesJune 2002 Question Paper 1Abed AymanNo ratings yet
- ISO/WD xxxxx-1: © ISO 2010 - All Rights ReservedDocument19 pagesISO/WD xxxxx-1: © ISO 2010 - All Rights ReservedAnanda Ganesh MadheswaranNo ratings yet
- Chem 321 Chapter 8 BDocument39 pagesChem 321 Chapter 8 BLin Xian XingNo ratings yet
- 5 6064450704775315719 PDFDocument6 pages5 6064450704775315719 PDFMriganga SarmaNo ratings yet
- Aromatic CompoundsDocument3 pagesAromatic CompoundsRonak GurJarNo ratings yet
- End of Unit Test: Name Class DateDocument4 pagesEnd of Unit Test: Name Class DateVictor Barber Sanchis100% (1)
- Chapter 19Document11 pagesChapter 19tegalNo ratings yet
- Determination of Residual Chlorine and Chlorine Demand: Break Point ChlorinationDocument22 pagesDetermination of Residual Chlorine and Chlorine Demand: Break Point Chlorinationনীল জোছনা100% (1)
- Jo 0503299Document6 pagesJo 0503299Kyucheol PaikNo ratings yet
- Sponification of Edible OilDocument2 pagesSponification of Edible OilUsman GhaniNo ratings yet
- CH 17Document54 pagesCH 17firebot4No ratings yet
- pH and Buffers LabDocument3 pagespH and Buffers LabFaye SaludNo ratings yet
- Chapter 4 ThermochemistryDocument15 pagesChapter 4 ThermochemistrySherry LeeNo ratings yet
- Boas Práticas para Titulação Karl Fischer - Metler ToledoDocument104 pagesBoas Práticas para Titulação Karl Fischer - Metler ToledoAnderson AzevedoNo ratings yet
- DynamicDocument34 pagesDynamicCentral HydraulicsNo ratings yet
- Mechanism and Kinetics of Ethanol Coupling To Butanol Over HydroxyapatiteDocument35 pagesMechanism and Kinetics of Ethanol Coupling To Butanol Over HydroxyapatiteNazar AbdimomunovNo ratings yet
- Sample Question Answers - Unit 4: Upon Successful Completion of This Unit, The Students Should Be Able ToDocument14 pagesSample Question Answers - Unit 4: Upon Successful Completion of This Unit, The Students Should Be Able TobillingsleyNo ratings yet
- IUPAC Name: AzaneDocument7 pagesIUPAC Name: Azaneمحمد خليلNo ratings yet
- Animal CareDocument12 pagesAnimal Caremirzaayan918No ratings yet
- Premium Shave Cream: Products Highlighted: Floraesters K-100 Jojoba and Floraesters 30Document1 pagePremium Shave Cream: Products Highlighted: Floraesters K-100 Jojoba and Floraesters 30zaryab khanNo ratings yet
- Acid BaseDocument34 pagesAcid Basehay0117No ratings yet
- Boron FamilyDocument12 pagesBoron FamilyGaurav DubeyNo ratings yet
- 14) Coordination Chemistry PDFDocument28 pages14) Coordination Chemistry PDFBj Larracas100% (6)
- US2478396 Activation of Cellulose For AcylationDocument3 pagesUS2478396 Activation of Cellulose For AcylationEetu SatosalmiNo ratings yet
- Cacl Ca: Seigfred John M. Miranda Che121.1 Laboratory 1Document5 pagesCacl Ca: Seigfred John M. Miranda Che121.1 Laboratory 1Kathleen Caryl PiedadNo ratings yet
- Phenol Jee MainsDocument9 pagesPhenol Jee MainsgetsugoshimuraNo ratings yet
- Transition Elements Chemistry ChapterDocument6 pagesTransition Elements Chemistry ChapterKashif MagsiNo ratings yet
- Organic Chemistry 5th Edition Brown Foote Iverson Anslyn Test BankDocument34 pagesOrganic Chemistry 5th Edition Brown Foote Iverson Anslyn Test Bankjames100% (25)