Professional Documents
Culture Documents
Stirring and Shrouding Gases
Uploaded by
akshukOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Stirring and Shrouding Gases
Uploaded by
akshukCopyright:
Available Formats
Selection of Stirring and Shrouding Gases for Steelmaking Applications
Dr. Ronald J. Selines Manager - Process Metallurgy, Linde Division, Union Carbide Corporation Tarrytown Technical Center Tarrytown, New York
Copyright 1988, Union Carbide Corporation
ABSTRACT Argon. nitrogen, carbon dioxide, and carbon monoxide are the gases used to provide stirring or shrouding in steelmaking applications. The behavior of these gases when in contact with molten steel is reviewed, and the criteria that can influence the selection of a particular gas are discussed in general. The specific issues associated with three representative applications; BOF stirring, billet caster shrouding, and ladle stirring are discussed in detail. INTRODUCTION The use of nitrogen and argon to provide stirring and protection from atmospheric contamination is widespread in steel meltshops, and resultant benefits are well documented. Recently, experience using carbon dioxide or carbon monoxide for such applications has been reported. The selection of the most appropriate gas for a specific application may not be straightforward and can involve consideration of a number of factors including steel chemistry and quality, injection device life, gas and overall process cost, and safety. This paper will review the relative merits of each gas with respect to each of the factors which can influence the selection process. Included is a description of the fundamental behavior of each gas in contact with molten steel and resultant consequences for specific steel melting, refining, and casting operations.
GAS CHARACTERISTICS Argon Argon is completely inert to molten steel. It provides stirring and a protective atmosphere with no potential for undesired reactions and no measurable solubility. Its only effect on steel chemistry is to remove dissolved hydrogen, oxygen, and nitrogen via a sparging mechanism. Figure 1 shows theoretical argon degassing requirements for nitrogen and hydrogen removal. Argon is also used as an inert diluting gas to promote carbon removal in Union Carbide's proprietary AOD process. Nitrogen Nitrogen has a solubility of 380 ppm in molten iron at 1530C, and its solubility increases with temperature. The presence of elements such as aluminum, titanium, vanadium, etc., further increases nitrogen solubility. Consequently, its use for stirring or shrouding can result in higher final nitrogen contents. The kinetics of nitrogen absorption via the reaction: N2(g) 2N (1)
are strongly influenced by the oxygen and sulfur content of the steel. These surface active elements retard nitrogen dissolution kinetics by preferentially occupying surface sites where reaction (1) would otherwise occur. Thus, highly desulfurized steels 1
and aluminum or calcium deoxidized steels are particularly prone to nitrogen pick-up. Figure 2 shows the effect of sulfur content on the kinetics of nitrogen absorption by iron droplets at 1600C.(1) Carbon Dioxide A thermodynamic analysis shows that carbon dioxide can react with iron, carbon or any deoxidizers which are present in the steel melt. Reactions which are thermodynamically favored are summarized in Table I. For applications where nondeoxidized baths are present, such as AOD and BOF converters, carbon dioxide can react with either carbon or iron to form FeO and/or carbon monoxide depending on carbon content. In deoxidized steels, carbon dioxide can react with the deoxidant to form the corresponding oxide and either dissolved carbon or carbon monoxide. The extent to which these reactions proceed is controlled by kinetic considerations. D. R. Sain et al(2) have studied the interfacial reaction kinetics of carbon dioxide with carbon in liquid iron as a function of temperature and pressure. The experimentally measured reaction rates are relatively high and suggest that bubbles of carbon dioxide will be rapidly consumed by steel melts via reaction with dissolved carbon assuming that mass transfer is not limiting (see Appendix I). T. Bruce et al(3) have considered carbon dioxide stirring of aluminum killed steels and conclude that aluminum diffusion in the bath is the rate controlling step. They report the absence of visible bubbles at low flow rates and up to a 50% decrease in injector life time as evidence that reactions to form A1203, FeO, and dissolved C do proceed to a significant extent. Linde's experience with carbon dioxide in AOD steelmaking confirms these conclusions. Specifically, complete reaction of carbon dioxide with carbon must be inferred to close an oxygen balance for the decarburization step. In addition, 2
stoichiometric increases in bath carbon content when blowing a mixture of oxygen and carbon dioxide into a bath containing aluminum are observed. Carbon Monoxide The use of carbon monoxide in Q-BOP and BOP furnaces has been recently reported.(4,5) However, there is little experience to date, and the behavior of carbon monoxide in the melt is not well understood. Effects attributable to low reactivity and a resultant high partial pressure of carbon monoxide as well as effects attributed to significant conversion to dissolved carbon and carbon dioxide via the reaction: 2CO C + CO2 (2)
are described. In general, the use of carbon monoxide for steelmaking applications is of reduced interest due to availability and safety considerations, and only its use for BOF stirring will be considered. SELECTION CRITERIA Having reviewed the fundamental behavior of argon, nitrogen, and carbon dioxide in molten iron and steel, this section will consider the major factors which can influence the selection of a gas for steelmaking applications. Criteria related to steel chemistry, quality, refractory plug wear, economics and safety are examined. A relative ranking of these gases for each of these categories is given in Table II, and a more detailed discussion follows. Steel Chemistry Since argon is totally inert, it is the gas of choice when ladle stirring or shrouding is required and changes in nitrogen, carbon, or deoxidant levels must be minimized. It is also used in AOD, vacuum processing, and ladle degassing when substantial
reductions in nitrogen, hydrogen, or oxygen content are desired, and when producing ultra-low carbon content grades (C <0.02%). Argon is also used for pneumatic injection of reagents for desulfurization and inclusion shape control. Nitrogen is used in applications with grades that can tolerate the associated nitrogen pick-up or with grades for which nitrogen alloying is desired. The amount of pick-up is controlled by the extent of contact with the molten metal, steel chemistry, and total amount of gas used. Typical results will be presented later when specific applications are discussed. Carbon dioxide is used for applications where nitrogen pick-up must be minimized and potential changes in carbon, silicon, or aluminum content can be tolerated. Quality While proper steel chemistry is fundamental to quality, gas selection may also affect other aspects of quality such as inclusion and pin-hole content of as-cast product. For example, excessive use of argon through submerged entry nozzles and gaseous argon shrouding of continuously cast billet can result in pin-holes believed to result from trapped insoluble argon bubbles.(6-7) Consequently, the use of argon for billet shrouding should be carefully evaluated to assess whether pin-holes are being formed and, if so, whether final product quality is being compromised. The reaction of carbon dioxide with silicon or aluminum in killed steels to form oxide inclusions and the possibility that these reaction products are retained in the final product represent a significant risk to product quality. For example, an increase in the macro-inclusion content of carbon dioxide vg~us argon or nitrogen shrouded continuouM cast billet has been reported.(6) Another study found no such increase.(7) However, the shroud atmospheres in this case contained an average of 2.5% oxygen 3
which is well above the recommended level of 0.5% and would likely mask any differences due to using carbon dioxide rather than argon. Refractory Plug Wear Many stirring applications feature gas injection through refractory plugs of various design and materials of construction. The wear rate of these plugs depends on several factors. While a review of all these factors is beyond the scope of this paper, it is appropriate to discuss the effect of gas type. In general, refractory plugs are not suitable for oxygen injection, and are used only to provide stirring. Several studies have concluded that the use of carbon dioxide results in accelerated plug wear compared to argon, nitrogen, or carbon monoxide. Up to a 50% decrease in plug life has been reported for iadle stirring,(3) and Figure 3 shows the effect of gas type and tem erature on the wear rate of a directed porosity plug used for BOF stirring. (8) In this case, the wear rate increased from 0.2 to 0.8 mm/charge when carbon dioxide was substituted for either argon or carbon monoxide. Such increases in wear rate will adversely affect overall refractory life, maintenance, vessel availability and stirring reliability. Economics The economic considerations which can influence gas selection include the unit gas cost, deoxidant consumption, and refractory element wear. While unit gas costs depend upon factors such as volume and location, for installations supplied by bulk cryogenic storage tanks, the cost of nitrogen and carbon dioxide are roughly equivalent, while argon is about eight times more expensive. The use of carbon dioxide for stirring or shrouding of killed steels will result in increased deoxidant consumption which may more than offset the savings which result from avoiding the use of argon. Similarly, lost benefits and savings which result from increased wear of refractory plugs can overshadow the savings which result from avoiding the use of argon for ladle or BOF stirring. Since the most cost
effective choice of gas usually requires consideration of several factors, more detailed analyses of these economic issues will be presented in the section of this paper which discusses specific applications. Safety The use of any of these gases introduces potential hazards, and equipment design, operating practices, and maintenance procedures must be established to reduce the liklihood and seriousness of potential accidents to acceptable levels. The most serious hazard associated with argon, nitrogen or carbon dioxide is asphyxiation in confined spaces due to lack of oxygen. In many situations, the relative severity of the hazard increases with increasing specific gravity (sg). Consequently. a ranking in order of increasing hazard would be; nitrogen (sg0.97), argon (sg-1.38), and carbon dioxide (sg1.52). It should also be noted that the reactivity of carbon dioxide in the blood stream results in a maximum eight hour exposure limit of 0.5% in air as recommended by the American Conference of Governmental Industrial Hygienists. An additional potential hazard associated with carbon dioxide use is the formation of carbon monoxide due to dissociation or reaction with iron, carbon or silicon. Carbon monoxide is flammable and toxic with a recommended maximum exposure limit of 50 ppm (ACGIH 1984-85). Consequently, the environment should be checked to assure safe levels for operations that require relatively high flow rates such as billet shrouding . Obviously, the use of carbon monoxide for BOF stirring poses a significantly greater hazard due to the large quantity of gas required and risk of explosion. APPLICATIONS This section will consider the issues which can impact gas selection for specific steelmaking applications. Since it is not possible to cover all uses, three applications have been selected which 4
are in widespread use and represent a significant portion of total inert gas use by the industry. BOF Stirring Pneumatic stirring provides a variety of benefits to BOF steelmaking and has been widely adopted throughout the world. Most common is a practice which uses nitrogen and argon, with final nitrogen and carbon levels dictating the relative amounts of each gas used. A BOF stirring practice with either carbon dioxide or carbon monoxide is used in several Japanese and European mills and in one US location. The gases are injected through tuyeres located in the bottom of the vessel. Tuyere design is usually either: concentric tubes to provide an annulus for gas injection; or multiple small diameter tubes or channels incorporated in a high quality carbon-magnesite block. Since this is an oxygen based decarburization process, the reactivty of carbon dioxide is not detrimental as long as decarburization to low levels is not required. In fact, the reaction of carbon dioxide with carbon to form carbon monoxide (See Table I) should decrease oxygen blowing times and consumptions slightly (less than 3%). However, as shown in Figure 3(8), dissolved oxygen contents at end of blow are significantly higher when using carbon dioxide rather than argon or nitrogen for carbon levels below 0.1%. In addition, as carbon content decreases in this range, the reaction of carbon dioxide with iron to form dissolved carbon as well as carbon monoxide (See Table I) will be increasingly favored, further hindering decarburization to low levels. Consequently, the amount of low carbon steel grades produced can significantly impact potential savings associated with the substitution of carbon dioxide for argon in this process. Its use can reduce the ability to achieve low carbon levels, and decreases in yield and refractory life associated with high oxygen and FeO contents will lead to higher refining costs. The other consideration affecting the use of carbon dioxide is its effect on tuyere wear rates. Figure 4
shows that tuyere wear rates are significantly accelerated when using carbon dioxide rather than argon or carbon monoxide. Examination of worn tuyeres indicates that this acceleration of wear rate is due to the formation of FeO at the tuyere which results in local 4ttack of the carbon-magnesite refractory as shown schematically in Figure 08) Such an increase in tuyere wear rate can result in premature loss of stirring and associated benefits, reduced vessel life and productivity, or both. An attempt to put some perspective on the tradeoff between gas cost and overall BOF operating costs is presented in Figure 6. The case considers a 230 ton BOF with a 1500 heat campaign life and treats the effect of carbon dioxide on tuyere wear rate as a variable. The basic assumption made is that any increase in tuyere wear rate will result in a corresponding decrease in the number of stirred heats but will not shorten overall vessel life. In other words, the consequences of premature loss of stirring translate into lost savings due to a lack of stirring on remaining production. The vessel remains in service for the full 1500 heats with no loss in productivity or refractories. The analysis shows that for the stated assumptions for relative gas costs and stirring cost benefits, a 25% increase in tuyere wear rate is the break even point. This break even point will shift depending on the relative magnitudes of cost premium for argon vs. carbon dioxide and overall cost savings associated with stirring, and an analysis based on actual costs and operating data is recommended. However, such an analysis does point out that the economic consequences of accelerated tuyere wear can offset the cost savings associated with substituting carbon dioxide for argon. A discussion of BOF stirring would not be complete without mentioning carbon monoxide. Published results indicate that, compared with argon stirring, slag FeO contents are unchanged, dissolved ox en contents are slightly higher, and tuyere wear rates are about equal. (4,5,8) Consequently, carbon monoxide appears to be an acceptable alternative 5
to argon on the basis of metallurgical and operational considerations. The troublesome aspects of carbon monoxide use are increased safety risk due to its toxicity and flammability and a requirement for additional capital equipment to reclaim the carbon monoxide from the BOF off-gas stream. Billet Casting Gas purging of stovepipe type shrouds is the usual method for protecting tundish to mold streams from atmospheric contamination. Elimination of large reoxidation type inclusions is the most common objective. A reduction in nitrogen pick-up may also be important. Due to visibility and accessibility requirements, stovepipe shrouds may have significant gaps or openings and typically require gas consumptions in the range of 50-200 ft3 (1.45.7m3) per ton. If prevention of reoxidation is the only need, nitrogen is the best gas choice. For best results, the shroud design and gas flow rates used should be capable of achieving oxygen levels below 1%, and preferably below 0.5% as measured within the stovepipe shroud during casting. The magnitude of nitrogen pick-up depends on steel chemistry and casting conditions and is usually in the 5 to 10 ppm range. Nitrogen sensitive grades, most notably boron containing steels or wire grades, are sometimes shrouded with argon to reduce the nitrogen pick-up which would be associated with gaseous nitrogen shrouding. Due to the high gas consumption required, the use of argon rather than nitrogen involves a significant cost increase, and carbon dioxide shrouding of nitrogen sensitive grades has been suggested as a more economic alternative. However, while carbon dioxide is as effective as argon in preventing nitrogen pick-up, there is a question regarding its ability to also prevent reoxidation.
Reoxidation occurs due to contact of the metal stream with the surrounding atmosphere and the entrainment and subsequent reaction (if any) of this atmosphere in the stream and, subsequently, the continuous casting mold itself as the stream impinges on the molten metal surface. Levitated drop experiments have been used to evaluate the kinetics of oxygen absorption in atmospheres containing 1-20% oxygen, nitrogen, and carbon dioxide, and the results are summarized in Figure 7. The data clearly show that the rate of oxygen absorption in pure carbon dioxide is significantly lower than in atmospheres containing one or more percent oxygen, and that nitrogen does indeed completely eliminate reoxidation. This data suggests that carbon dioxide shrouding should offer an intermediate level of protection from reoxidation compared to argon or nitrogen. Results from commercial trials comparing the relative performance of nitrogen, argon, and carbon dioxide are not consistent and controversy concerning the degree of protection afforded by carbon dioxide remains. Figure 8 shows typical results from tests at Auburn Steel using an enclosed shroud with residual oxygen contents less than 0.2%.(6) The data show that carbon dioxide shrouded billets contain significantly more large reoxidation inclusions compared to nitrogen or argon shrouded material, and, surprisingly, compared to unshrouded material as well. This last result may be due to the protective atmosphere formed by partial combustion of the mold lubricant by air which is lost when shrouding with carbon dioxide. Figure 9 shows typical results from tests at CF&I Steel using a shroud design which usulted in residual oxygen contents of 1-11% (2.5% average)(7). These results show comparable levels of cleanliness in argon compared to carbon dioxide shrouded material. However, in view of the data presented in Figure 7, one may speculate that it was the residual oxygen levels present with both gases that was 6
controlling the overall extent of reoxidation. In view of the importance of preventing reoxidation, the use of carbon dioxide is not recommended due to its questionable performance in this regard. While argon does offer the best protection against both reoxidation and nitrogen pick-up, it appears that it can result in an increase in pin-hole content. Both of the tests referred to above report such an effect. One may speculate that such an effect is due to the physical entrapment of insoluble and nonreactive argon bubbles, and that solidification conditions control their occurence. In any event, it is recommended that argon shrouded billets be carefully evaluated to assess whether pin-hole content remains at acceptable levels. Ladle Stirring Ladle stirring is commonly practiced to aid desulfurization, homogenize temperature and composition, remove inclusions, assist vacuum degassing, etc. Gas is usually injected through a porous refractory stirring element located in the bottom of the ladle or through a refractory coated lance. Gas injection rates are usually less than 10 scfm (0.29 m3/min). and gas consumptions are usually about 1 ft3/ton (0.03 m3/ton). Since most ladle stirring is performed to further improve the quality of deoxidized steel, argon is the gas most often selected for this application. The required stirring is provided while minimizing possible adverse reactions. Argon also provides good stirring element life. Nitrogen is used when the associated increase in nitrogen content can be tolerated. The increase in nitrogen content de ends on the steel chemistry and amount of nitrogen used. Hagerty et al (6) reported an increase in the 10 to 20 ppm range for 10 to 15 minutes of nitrogen stirring for AISI 1016 and 1035M grades containing 0.04% sulfur. In view of the potential for significant increases in nitrogen contents, the small added cost associated with argon can often be justified.
The use of carbon dioxide for ladle stirring has also been evaluated.(3) However, there are a number of considerations which make it a poor choice for this application as well. Since ladle stirring operations often involve deoxidized melts, the potential reactions of carbon dioxide with aluminum or silicon to form the corresponding oxide can compromise final product quality. While most of the oxide inclusion reaction products appear to be eliminated due to the stirring action, the possiblility of some amount carrying through to the final product will always exist. The reported decrease in porous refractory element life is of course another negative aspect associated with its use. Finally, it is not at all clear that the use of carbon dioxide rather than argon results in any overall cost savings. The reported observation that, Under certain conditions of injection, no bubbles seem to reach the surface. suggests that reaction with aluminum is proceeding to near completion. The analysis in Table III shows that the value of the deoxidant consumed far exceeds the potential savings in gas costs for aluminum killed grades. If it is likewise assumed that the reaction of carbon dioxide proceeds to near completion in silicon killed grades, then the value of the lost silicon is comparable to the potential savings in gas costs. Such an analysis can also be applied to the billet casting application which would also involve deoxidized steel. However, in this case, it is difficult to estimate how much of the total carbon dioxide introduced into the shroud reacts to consume deoxidant. CONCLUSIONS A review of the behavior of the gases used to provide stirring and atmosphere protection in steelmaking has shown that argon alone is completely non-reactive. Consequently, it is the gas of choice for applications that require the best possible quality and the least possible change in steel chemistry. The only exception to this conclusion is its use for shrouding on continuous casters where a possibility of increased pinhole content exists. Since argon is denser than air, it is 7
particularly effective for purging molds and is widely used to improve quality in ingot teeming operations. However, this property also increases the risk of asphyxiation, and special precautions must be taken when entering confined spaces. Argon is the most expensive of the gases normally used for such applications, and nitrogen, carbon dioxide, and carbon monoxide are substituted when possible. Nitrogen is used whenever the associated increase in nitrogen content can be tolerated. BOF stirring, AOD refining, billet caster shrouding, and ladle stirring are typical applications. However, the relatively small cost savings associated with ladle stirring may not justify the resultant increase in nitrogen content. Nitrogen is less dense than air and consequently is not an efficient gas for mold purging and is less likely to introduce asphyxiation hazards. Carbon dioxide can be substituted for nitrogen or argon to reduce nitrogen pick-up or gas cost respectively. However, there are several undesirable characteristics associated with its use which should be considered in order to fully assess overall suitability. Carbon dioxide can undergo appreciable reaction when in contact with molten steel, thereby changing steel chemistry and possibly increasing inclusion content. Its use can also increase the rate of wear of gas injection tuyeres. The reactivity of carbon dioxide is of less concern in oxygen based applications such as BOF and AOD refining unless ultra-low carbon contents are required, and these are the processes in which its use may be justified. The potential for reaction with deoxidant in killed steels and associated formation of oxide inclusions make its use for shrouding or ladle stirring applications questionable from both quality and overall economic viewpoints. Carbon dioxide is also denser than air and poses increased risk of asphyxiation in confined spaces. There may also be a potential for appreciable levels of carbon monoxide associated with its use. Carbon monoxide is being used for BOF stirring by a few Japanese steelmakers. It appears to be an
excellent substitute for nitrogen and argon from both metallurgical and operational viewpoints. However, since bulk quantities of carbon monoxide are not available commercially, it must be recovered from the BOF off-gas stream, and overall economics can be strongly influenced by capital requirements and the value of any remaining carbon monoxide as a fuel gas. In addition, there are obvious safety considerations associated with the storage, handling and use of such large quantities of a gas which is both toxic and flammable. REFERENCES (1) L.A.Greenberg and A.McLean, Nitrogen Pick-up in Low Sulfur Steel, Ironmaking and Steelmaking, 9, 2, 1982, pp.58. (2) D.R.Sain and G.R.Belton, Interfacial Reaction Kinetics in the Decarburization of Liquid Iron by Carbon Dioxide, Met.Trans. B, Vol. 7B, June 1976, p. 235. (3) T.Bruce et al, Effects Of CO2 Stirring in a Ladle, Electric Furnace Conference Proceedings, Vol. 45, Chicago, IL, 1987, pp. 293-297. (4) T.Sakuraya et al, Protection of Oxygen Bottom Blown Tuyeres by CO Gas, Steelmaking Conference Proceedings, Washington, DC, Vol. 69, 1986, pp. 639-646. (5) H.Yamana et al, CO Gas Bottom Blowing in the Top and Bottom Blowing Converter, Ironmaking and Steelmaking, Steelmaking Conference Proceedings, Pittsburgh, PA, Vol. 70, 1987, pp. 339-346. (6) L.J.Hagerty and J.A.Rossi, Shrouding of Continuous Billet Castings at Auburn Steel with Argon, Nitrogen and Carbon Dioxide, Electric Furnace Conference Proceedings, Vol. 44, Dallas, TX, 1986, pp. 153-159. (7) C.T.Jensen et al, Atmospheric Protection of Billet Streams Using Carbon Dioxide, Electric
Furnace Conference Proceedings, Vol. 45, Chicago, IL, 1987, pp. 57-63. (8) Rinsing Effect of LD-KGC Process, Kawasaki Steel Corporation, private communication. (9) M.Nishi et al, Development of the Multiple Hole Plug for Top and Bottom Blown Converter, Nippon Kokan KK, private communication.
TABLE I - CO2 REACTIONS Non-Deoxidized Carbon Steels CO2 + C 2CO CO2 + Fe CO + FeO Deoxidized Steels 3CO2 + 4Al 2Al203 + 3C 2CO2 + Si SiO 2 + 2CO
TABLE II - QUALITATIVE RANKING OF GAS TYPES FOR SEVERAL SELECTION CRITERIA SELECTION CRITERIA Steel Quality Low Low Gas Inclusions Pinholes Cost + + NA + + NA + + SD
Gas Type Ar N2 CO2 CO
Steel Chemistry Low High Low N N O + + + + + + 0
Cost Plug Wear + + 0
Safety# Deox. Use + + NA 0 + 0 -
0 + # NA ND SD
Poor Acceptable Recommended See text for safety information for all gases Not applicable - used for BOF stirring only Not determined Site dependent - recovered from BOF off-gas
TABLE III - ECONOMICS OF CARBON DIOXIDE STIRRING DEOXIDIZED STEELS
BREAK EVEN GAS COST DIFFERENTIAL* $/100 ft3 ($/m3) 7.00 2.40 (2.48) (0.85)
STEEL TYPE Al Deoxidized Si Deoxidized
*
REACTION 3CO2 + 4Al 2Al2O3 + 3C 2CO2 + Si SiO2 + 2CO
DEOXIDANT CONSUMPTION lbs/ft3 of CO2 (kg/m3 Of CO2) 0.093 0.036 (1.49) (0.58)
Assumes $0.75/lb Aluminum, $0.65/lb Silicon and complete reaction of carbon dioxide.
FIGURE CAPTIONS 1. Theoretical argon requirement for removal of hydrogen or nitrogen from molten steel at 2912F. 2. Effect of sulfur content on nitrogen pick-up: (a) increase in nitrogen content vs. time for levitated droplets with varying sulfur contents; (b) effect of sulfur content on the rate of nitrogen pick-up by levitated droplets.(1) 3. Effect of carbon dioxide compared to argon or nitrogen stirring on bath oxygen content at the end of oxygen blowing in the BOF.(8) 4. Effect of carbon dioxide compared to argon or nitrogen stirring on the wear rate of BOF stirring elements.(8) 5. Schematic illustration of the mechanism of increased element wear in the BOF due to carbon dioxide stirring and associated formation of FeO which locally attacks the magnesite-carbon refractory element.(9) 6. Relationship between increased refractory wear due to carbon dioxide use and overall savings due to BOF stirring assuming an inverse linear relation between increased tuyere wear rate and percentage of heats stirred for a campaign. 7. Effect of oxygen-nitrogen mixtures, pure nitrogen, and carbon dioxide on the variation of the n9en content of levitated steel droplets weighing approximately one gram.(7) 8. Effect of nitrogen, argon, or carbon dioxide shrouding on the macro-inclusion content of grade 1016 billet.(6) (Shroud oxygen content less than 0.2%.) 9. Effect of argon or carbon dioxide shrouding on the macro-inclusion content of grade SAE J422a billet.(7) (Average shroud oxygen content about 2.5%.)
10
APPENDIX I - REACTION OF A CARBON DIOXIDE BUBBLE IN A STEEL MELT
REACTION: CO2 + C 2CO (Rate Constant K = 3.16 x 10-4 mole/cm2/sec/atm2) ASSUMPTIONS: Average bubble radius (R) = 5cm Initial CO2 pressure (PCO2 i) = 1.75 atm Average CO2 pressure ( PCO2 ) = 0.5 atm Temperature (T) = 1900K Initial CO2 content of bubble (ni CO2)
i PCO2 4 3R 3 RT 1.75 atm 522 cm 3 = cm 3 atm 82.05 1900 K mol k = 6 10 3 mole
Rate Of CO2 depletion
= K bubble surface area PCO2 = K 4R 2 PCO2 = 5 10 2 mole/ sec
Complete bubble reaction time (tR)
6 103 mole 5 102 mole / sec = 0.12 seconds =
11
FIGURE 1: Theoretical argon requirement for removal of hydrogen or nitrogen from molten steel at 2912F.
12
FIGURE 2: Effect of sulfur content on nitrogen pick-up: (a) increase in nitrogen content vs. time for levitated droplets with varying sulfur contents; (b) effect of sulfur content on the rate of nitrogen pick-up by levitated droplets.(1)
13
FIGURE 3: Effect of carbon dioxide compared to argon or nitrogen, stirring on bath oxygen content at the end of oxygen blowing in the BOF.(8)
14
FIGURE 4: Effect of carbon dioxide compared to argon or nitrogen stirring on the wear rate of BOF stirring elements.(8)
15
FIGURE 5: Schematic illustration of the mechanism of increased element wear in the BOF due to carbon dioxide stirring and associated formation of FeO which locally attacks the magnesite-carbon refractory element.(9)
16
FIGURE 6: Relationship between increased refractory wear due to carbon dioxide use and overall savings due to BOF stirring assuming an inverse linear relation between increased tuyere wear rate and percentage of heats stirred for a campaign.
17
FIGURE 7: Effect of oxygen-nitrogen mixtures, pure nitrogen, and carbon dioxide on the variation of the oxygen content of levitated steel droplets weighing approximately one gram.(7)
18
FIGURE 8: Effect of nitrogen, argon or carbon dioxide shrouding on the macro-inclusion content of grade 1016 billet.(6) (Shroud oxygen content less than 0.2%).
19
FIGURE 9: Effect of argon or carbon dioxide shrouding on the macro-inclusion content of grade SAE J422a billet.(7) (Average shroud oxygen content about 2.5%).
20
You might also like
- Biomass As Blast Furnace Injectant - Considering AvailabilityDocument10 pagesBiomass As Blast Furnace Injectant - Considering AvailabilityakshukNo ratings yet
- AISTech 2019 Successful Use Case Applications of Artificial Intelligence in The Steel IndustryDocument14 pagesAISTech 2019 Successful Use Case Applications of Artificial Intelligence in The Steel IndustryakshukNo ratings yet
- The Extractive Metallurgy of Zinc - by Roderick J. Sinclair: January 2006Document4 pagesThe Extractive Metallurgy of Zinc - by Roderick J. Sinclair: January 2006akshukNo ratings yet
- Physicochemical Aspects of Reactions in Ironmaking and Steelmaking Processes PDFDocument21 pagesPhysicochemical Aspects of Reactions in Ironmaking and Steelmaking Processes PDFakshukNo ratings yet
- Aparajita Stotra From Visnudharmottara P PDFDocument7 pagesAparajita Stotra From Visnudharmottara P PDFakshukNo ratings yet
- A Gibbs Energy Minimization Method For CDocument13 pagesA Gibbs Energy Minimization Method For CakshukNo ratings yet
- Part-1 Kinetics of ZNS RoastingDocument9 pagesPart-1 Kinetics of ZNS RoastingakshukNo ratings yet
- Urban Et Al. De-P Startegies Iron Steel Tech 2015Document12 pagesUrban Et Al. De-P Startegies Iron Steel Tech 2015akshukNo ratings yet
- Part-2 Kinetics of ZNS RoastingDocument7 pagesPart-2 Kinetics of ZNS RoastingakshukNo ratings yet
- Dri Update May - 2021Document31 pagesDri Update May - 2021Vivek RanganathanNo ratings yet
- Final Master ProgrammeDocument9 pagesFinal Master ProgrammeakshukNo ratings yet
- Overview of The Applications of Thermodynamic Databases To Steelmaking ProcessesDocument31 pagesOverview of The Applications of Thermodynamic Databases To Steelmaking ProcessesakshukNo ratings yet
- Ironmaking and Steelmaking PDFDocument466 pagesIronmaking and Steelmaking PDFakshukNo ratings yet
- Steelmaking Technology For The Last 100 Years TowaDocument32 pagesSteelmaking Technology For The Last 100 Years TowaHari BudiartoNo ratings yet
- Kinetics of Fundamental Reactions Pertinent To Steelmaking PDFDocument16 pagesKinetics of Fundamental Reactions Pertinent To Steelmaking PDFakshukNo ratings yet
- Ironmaking and Steelmaking PDFDocument466 pagesIronmaking and Steelmaking PDFakshukNo ratings yet
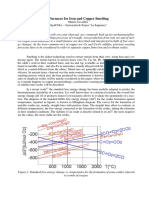
- Fe and Cu Smelting PDFDocument6 pagesFe and Cu Smelting PDFakshukNo ratings yet
- Climate Change and MigrationDocument9 pagesClimate Change and MigrationakshukNo ratings yet
- Manuscript IJCMR PDFDocument35 pagesManuscript IJCMR PDFakshukNo ratings yet
- Copper Extraction Mass Balance ProblemsDocument6 pagesCopper Extraction Mass Balance ProblemsakshukNo ratings yet
- NumericsAndOpenFOAMv7 LimitedDocument155 pagesNumericsAndOpenFOAMv7 LimitedakshukNo ratings yet
- The Phosphorus Reaction in Oxygen Steelmaking - Thermodynamic Equi PDFDocument211 pagesThe Phosphorus Reaction in Oxygen Steelmaking - Thermodynamic Equi PDFakshukNo ratings yet
- Climate Change and Migration PDFDocument287 pagesClimate Change and Migration PDFakshukNo ratings yet
- Iron and SteelmakingDocument139 pagesIron and SteelmakingRajatSehgalNo ratings yet
- Single-Step Ironmaking From Ore To Improve EnergyDocument134 pagesSingle-Step Ironmaking From Ore To Improve EnergyakshukNo ratings yet
- Stoichiometric Calculations for Combustion AnalysisDocument47 pagesStoichiometric Calculations for Combustion AnalysisHandayani KesumadewiNo ratings yet
- 19660004844Document244 pages19660004844akshukNo ratings yet
- BREF - Iron and Steel Production PDFDocument621 pagesBREF - Iron and Steel Production PDFRajeev SinghNo ratings yet
- Virtual+steelmaking Steeluniversity ENDocument16 pagesVirtual+steelmaking Steeluniversity ENakshukNo ratings yet
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5783)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (890)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (72)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- PEB Requirment by ClientDocument4 pagesPEB Requirment by ClientViraj ModiNo ratings yet
- Chapter 1 - The Empirical Beginnings and Basic Contents of Educational PsychologyDocument9 pagesChapter 1 - The Empirical Beginnings and Basic Contents of Educational PsychologyJoshua Almuete71% (7)
- Design Process at LEGODocument5 pagesDesign Process at LEGOkapsarcNo ratings yet
- Readme cljM880fw 2305076 518488 PDFDocument37 pagesReadme cljM880fw 2305076 518488 PDFjuan carlos MalagonNo ratings yet
- Double Burden of Malnutrition 2017Document31 pagesDouble Burden of Malnutrition 2017Gîrneţ AlinaNo ratings yet
- Color TheoryDocument28 pagesColor TheoryEka HaryantoNo ratings yet
- Description MicroscopeDocument4 pagesDescription MicroscopeRanma SaotomeNo ratings yet
- CP R77.30 ReleaseNotesDocument18 pagesCP R77.30 ReleaseNotesnenjamsNo ratings yet
- Fancy YarnsDocument7 pagesFancy Yarnsiriarn100% (1)
- Pump IntakeDocument6 pagesPump IntakeAnonymous CMS3dL1T100% (1)
- Adapting Cognitive Behavioral Techniques To Address Anxiety and Depression in Cognitively Able Emerging Adults On The Autism SpectrumDocument3 pagesAdapting Cognitive Behavioral Techniques To Address Anxiety and Depression in Cognitively Able Emerging Adults On The Autism SpectrumVini PezzinNo ratings yet
- Calibration GuideDocument8 pagesCalibration Guideallwin.c4512iNo ratings yet
- Morpho Full Fix 2Document9 pagesMorpho Full Fix 2Dayu AnaNo ratings yet
- Case Study On Maruti 800Document4 pagesCase Study On Maruti 800Nizar MesaniNo ratings yet
- About Topsøe - and What We DoDocument20 pagesAbout Topsøe - and What We DoAbhishek ChaudharyNo ratings yet
- SLU Missalette 2016 Capping (Not-Final)Document18 pagesSLU Missalette 2016 Capping (Not-Final)Teanu Jose Gabrillo TamayoNo ratings yet
- Tipolo WH Gantt ChartDocument15 pagesTipolo WH Gantt ChartMayeterisk RNo ratings yet
- FAI - Assignment Sheet (Both Assignments)Document5 pagesFAI - Assignment Sheet (Both Assignments)Wilson WongNo ratings yet
- Chick Lit: It's not a Gum, it's a Literary TrendDocument2 pagesChick Lit: It's not a Gum, it's a Literary TrendspringzmeNo ratings yet
- Sukkur IBA University Aptitude Test Candidates ListDocument306 pagesSukkur IBA University Aptitude Test Candidates ListFurkan memonNo ratings yet
- DOW™ HDPE 05962B: High Density Polyethylene ResinDocument3 pagesDOW™ HDPE 05962B: High Density Polyethylene ResinFredo NLNo ratings yet
- 2018 Diesel TOYOTA Jun11Document90 pages2018 Diesel TOYOTA Jun11eko sulistyo75% (4)
- Optitex Com Products 2d and 3d Cad SoftwareDocument12 pagesOptitex Com Products 2d and 3d Cad SoftwareFaathir Reza AvicenaNo ratings yet
- Sr. No. Name Nationality Profession Book Discovery Speciality 1 2 3 4 5 6 Unit 6Document3 pagesSr. No. Name Nationality Profession Book Discovery Speciality 1 2 3 4 5 6 Unit 6Dashrath KarkiNo ratings yet
- Otis Brochure Gen2life 191001-BELGIUM SmallDocument20 pagesOtis Brochure Gen2life 191001-BELGIUM SmallveersainikNo ratings yet
- Lorain Schools CEO Finalist Lloyd MartinDocument14 pagesLorain Schools CEO Finalist Lloyd MartinThe Morning JournalNo ratings yet
- MAN 2 Model Medan Introduction to School Environment ReportDocument45 pagesMAN 2 Model Medan Introduction to School Environment ReportdindaNo ratings yet
- Project Cost ForecastDocument11 pagesProject Cost ForecastJames MendesNo ratings yet
- Kashmira Karim Charaniya's ResumeDocument3 pagesKashmira Karim Charaniya's ResumeMegha JainNo ratings yet
- How To Use Hyper-V Snapshot Revert, Apply, and Delete OptionsDocument15 pagesHow To Use Hyper-V Snapshot Revert, Apply, and Delete OptionsKaran MishraNo ratings yet