Professional Documents
Culture Documents
Best Cases From The Afip
Uploaded by
api-148100258Original Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Best Cases From The Afip
Uploaded by
api-148100258Copyright:
Available Formats
AFIP ARCHIVES
843
RadioGraphics
Best Cases from the AFIP
Hemimegalencephaly1
Editors Note.Everyone who has taken the course in radiologic pathology at the Armed Forces Institute of Pathology (AFIP) remembers bringing beautifully illustrated cases for accession to the Institute. In recent years, the staff of the Department of Radiologic Pathology has judged the best cases by organ system, and recognition is given to the winners on the last day of the class. With each issue of RadioGraphics, one or more of these cases are published, written by the winning resident. Radiologicpathologic correlation is emphasized, and the causes of the imaging signs of various diseases are illustrated.
David D. Broumandi, MD Ulrike M. Hayward, MD James M. Benzian, MD Ignacio Gonzalez, MD Marvin D. Nelson, MD History
A 9-lb 13-oz full-term male was delivered by cesarean section due to failure of labor to progress. He was born to a diabetic but otherwise healthy mother and at birth had spells of apnea, which were attributed to seizures. Computed tomography (CT) of the brain revealed a possible mass in the right hemisphere, and magnetic resonance (MR) imaging suggested right hemimegalencephaly. Although initially responsive to antiepileptic treatments, during the following several months the patient continued to experience seizures despite optimal trials of phenobarbital and Topamax (topiramate; Ortho-McNeil Pharmaceutical, Raritan, NJ). Serial electroencephalograms demonstrated continued right-sided epileptiform activity with minimal involvement of the left frontal region. At ten months of age, the patient was developmentally delayed and had an enlarged head circumference measurement of 47.4 cm. The patient had no focal neurologic decits, and no cutaneous abnormalities were noted.
Imaging Findings
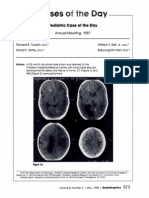
At 10 months of age, the patient underwent follow-up MR imaging of the brain, which showed marked enlargement of the right cerebral hemisphere and ndings consistent with hemimegalencephaly (Fig 1). The right cerebrum was diffusely dystrophic with broadened gyri and a diffusely thickened cortical mantle, consistent with areas of pachygyria and polymicrogyria. The right lateral
Index terms: Brain, abnormalities, 10.144 Brain, enlarged, 10.144 Brain, volume, 10.144 RadioGraphics 2004; 24:843 848 Published online 10.1148/rg.243035135
1From
the Department of Radiology, Santa Barbara Cottage Hospital, Pueblo at Bath St, 0689, Santa Barbara, CA 93102-0689 (D.D.B., U.M.H., J.M.B.); and the Departments of Pathology (I.G.) and Radiology (M.D.N.), Childrens Hospital, Los Angeles, Calif. Received May 19, 2003; revision requested June 18 and received July 16; accepted July 17. Address correspondence to D.D.B. (e-mail: dbroumandi@sbch.org). RSNA, 2004
844
May-June 2004
RG f Volume 24
Number 3
RadioGraphics
Figure 1. Axial unenhanced (a) and contrast material enhanced (b) T1-weighted MR images show enlargement of the right cerebral hemisphere, cavitation in the region of the centrum semiovale (arrowhead), and diffuse gyral thickening (arrows) with diminished sulcation, a nding consistent with pachygyria. There are patchy, linear regions of increased signal intensity in the white matter of the right hemisphere. No pathologic enhancement is seen on the contrast-enhanced image (b).
Figure 2. (a) Axial unenhanced T1-weighted MR image obtained at the level of the basal ganglia shows an enlarged and dysmorphic right cerebral hemisphere. The right basal ganglia are poorly demonstrated. There is moderate mass effect anteriorly (arrow). (b) On a sagittal T1-weighted MR image obtained at the midline, the corpus callosum is poorly seen (arrowhead).
ventricle was dysmorphic with portions that were compressed (Figs 2, 3). The right globus pallidus, putamen, and thalamus were poorly demarcated, and the corpus callosum was small (Fig 2). The
cerebellum and brainstem appeared normal (Fig 3). The left cerebral hemisphere had a more normal gyral pattern; however, there were regions of thick gray matter and pachygyria in the left frontal lobe, consistent with cortical dysplasia. The imaging ndings were consistent with right hemimegalencephaly.
RG f Volume 24
Number 3
Broumandi et al
845
RadioGraphics
Figure 3. Axial (a) and coronal (b) unenhanced T2-weighted MR images show enlargement of the right cerebral hemisphere. There is diffuse high signal intensity in the white matter, which correlates with histopathologic ndings of poor myelination and early cystic changes. The right lateral ventricle is compressed (arrow in b). The cerebellum is symmetrical and appears normal (arrowhead in b). Figure 4. Coronal section through the right frontal hemisphere (same orientation as in Fig 3b) shows broad gyri and a thick cortex, particularly in the frontal lobe (solid straight arrow). The occipital lobe has a more normal gyral pattern (arrowhead). The white matter is gliotic and shows areas of mucinous and cystic degeneration (curved arrow). The gray matter white matter junction is indistinct. The basal ganglia and thalami are small and poorly demarcated. Subventricular gray matter heterotopia is also noted (open arrow).
Pathologic Evaluation
Owing to the patients intractable seizures, a right hemispherectomy was performed for seizure control and prevention of progressive damage to the left hemisphere. The right hemisphere weighted 720 g. There was a diffusely abnormal gyral pattern with broad simple gyri and focal nodularity on the surface. Serial coronal sections revealed
short sulci and an irregularly thickened cortical band (Fig 4). There was a lateral sulcus that appeared short and markedly thickened, representing the sylvian ssure. The white matter was soft with friable cavitary regions. The right basal ganglia and thalami were poorly circumscribed. The cerebral commissures and internal capsule were thin. The ventricular system was compressed, and the ventricular wall was friable. Focal subventricular gray matter heterotopias were noted (Fig 4). The hippocampal formation was not distinguishable. In the occipital lobe, there were large areas of normal brain with intact architecture. Histologic sections revealed mild leptomeningeal brosis. The cortex was markedly thickened and dysplastic with poor lamination, subpial gliosis,
846
May-June 2004
RG f Volume 24
Number 3
RadioGraphics
Figure 5. (a) Low-power photomicrograph (hematoxylin-eosin stain) of the cerebral cortex shows a thickened cortex with poor neuronal lamination (between brackets). An increased number of neurons are present in the subcortical white matter (arrow). Large abnormal blood vessels with prominent perivascular spaces are also present in the white matter (arrowheads). (b) High-power photomicrograph (hematoxylin-eosin stain) shows poorly myelinated white matter containing scattered ectopic neurons (solid straight arrow), gliosis with hypertrophic changes (curved arrow), numerous Rosenthal bers (arrowheads), and vacuolar changes in the white matter. Focally scattered calcications are also present in the white matter (open arrow).
and scattered large neurons, many showing abnormal orientation (Fig 5). Focal areas with fusion of the molecular layers of adjacent gyri were present. The white matter was poorly myelinated and contained numerous scattered ectopic neurons, clusters and rows of hypertrophic astrocytes, and Rosenthal bers, represented by thick, elongated, brightly eosinophilic structures in a predominantly perivascular distribution (Fig 5). Rosenthal bers are found in conditions of longstanding gliosis, occasional tumors, and rare metabolic degenerative disorders. Areas of marked white matter vacuolation and cystic changes of varying degree were also noted. The histopathologic ndings conrmed the diagnosis of right hemimegalencephaly.
Discussion
Hemimegalencephaly or unilateral megalencephaly is a congenital disorder in which there is hamartomatous overgrowth of all or part of a cerebral hemisphere (1,2). The affected hemisphere may have focal or diffuse neuronal migration defects, with areas of polymicrogyria, pachygyria, and heterotopia. Hemimegalencephaly is a rare
disorder and was rst described by Sims in 1835 after reviewing 253 autopsies (3). Although the cause is unknown, it is postulated that it occurs due to insults during the second trimester of pregnancy, or as early as the 3rd week of gestation, as a genetically programmed developmental disorder related to cellular lineage and establishment of symmetry (1). Hemimegalencephaly may also be considered a primary disorder of proliferation wherein the neurons that are unable to form synaptic connections are not eliminated and are thus accumulated. Hemimegalencephaly differs from other cerebral dysgeneses because of its extreme asymmetry not corresponding to any normal stage of human brain development. No chromosomal abnormalities have been associated with hemimegalencephaly. There are three types of hemimegalencephaly (1). The isolated form, as in our case, occurs as a sporadic disorder without hemicorporal hypertrophy or cutaneous or systemic involvement. The syndromic form is associated with other diseases and may occur as hemihypertrophy of part or all of the ipsilateral body. It has been described in patients with epidermal nevus syndrome, Proteus syndrome, neurobromatosis type 1, hypermelanosis of Ito, Klippel-WeberTrenaunay syndrome, and tuberous sclerosis (2,4). Therefore, the syndromic type may follow a
RG f Volume 24
Number 3
Broumandi et al
847
RadioGraphics
mendelian pattern of inheritance. The third and least common type is total hemimegalencephaly, in which there is also enlargement of the ipsilateral half of the brainstem and cerebellum. Affected patients may have macrocephaly at birth and in early infancy and often present with an intractable seizure disorder, hemiplegia, and severe developmental delay (2). Males and females are equally affected. Pregnancy is usually uncomplicated, but cesarean section may be required owing to cephalopelvic disproportion. Therefore, macrocephaly is often the rst presentation at birth (1). Hemimegalencephaly has a high mortality in infancy unrelated to surgery (5,6). A brain tumor may be suspected when there is rapid enlargement of the head in the rst months of life. Patients have been misdiagnosed as having obstructive hydrocephalus and undergone unnecessary ventriculoperitoneal shunting (1). In hemimegalencephaly, the clinical signs of intracranial hypertension such as separation of sutures, bulging fontanels, and the setting-sun sign of the eyes are absent. The latter is characteristic of increased intracranial pressure in infants, with both ocular globes deviated downward, the upper lids retracted, and the white sclerae visible above the iris. Epilepsy is the most frequent neurologic manifestation, occurring in greater than 90% of patients (1). Although progressive hemiplegia and hemianopia are common, some patients do not have focal motor decits (1). Hemimegalencephaly in association with neurobromatosis type 1 may be associated with a more favorable clinical course (2,4). The diagnosis of hemimegalencephaly can usually be made at cross-sectional imaging. At CT, asymmetry of the cranium may be evident with enlargement of all or part of a cerebral hemisphere and ipsilateral ventricle. There is often focal, small, or extensive calcication in the white and gray matter, and the white matter may have abnormally low attenuation representing heterotopia and dysplasia of neurons. MR is the imaging modality of choice. A characteristic nding is straightening of the ipsilateral frontal horn of the enlarged ventricle (2). However, the ipsilateral ventricle may be small in some patients. In this case, ventricular enlargement is less severe compared with that of the involved hemisphere. At MR imaging, the white matter shows heterogeneous but frequently high signal intensity and there is often distinction of areas of agyria, pachygyria, and/or polymicrogyria. The white
matter of the affected hemisphere may show advanced myelination for age (7). There is a roughly inverse relationship between the severity of the cortical and white matter abnormalities and the size of the cerebral hemisphere. Patients with agyria tend to have mild to moderate hemispheric enlargement, while those with polymicrogyria have more severe hemispheric enlargement (2,4). Prenatal and postnatal cranial sonography may reveal ventricular asymmetry and unilateral ventricular dilatation. Functional imaging with positron emission tomography has had good correlation with CT and MR imaging ndings and has disclosed functionally abnormal brain regions in the noninvolved hemisphere that appeared structurally normal at CT and MR imaging (8). The gross pathologic appearance correlates with the imaging ndings of enlargement of the affected cerebral hemisphere. The brain surface may show pachygyria and polymicrogyria. Microscopically, nerve cells are larger and less densely packed than in the normal side of the brain, and the number of glial cells is increased. Areas of polymicrogyria, neuronal heterotopia, and pachygyria occur. Histologically, there is no difference between focal cortical dysplasia and hemimegalencephaly. However, macroscopically, hemimegalencephaly involves the whole hemisphere, whereas focal cortical dysplasia is more limited (9). White matter may show areas of poor myelination, cystic change, and gliosis, which correspond to increased signal intensity on T2weighted MR images. Our patient had extensive gliosis, microcystic changes, and Rosenthal bers in the white matter resembling leukodystrophy. Such extensive white matter involvement is unusual in hemimegalencephaly. Delayed myelination was the extent of involvement described by Woo et al (10) in three patients with hemimegalencephaly. Syndromic hemimegalencephaly has a worse prognosis than the isolated type, and there is generally poor neurologic function in cases of hemimegalencephaly. Seizure control is the principal goal of therapy, and patients often require multiple antiepileptic medications that have adverse side effects. Hemispherectomy was rst performed for treatment of refractory epilepsy in 1978 and is considered the best therapeutic choice for patients with intractable seizures (5,6).
848
May-June 2004
RG f Volume 24
Number 3
RadioGraphics
Anatomic or functional hemispherectomy has also been performed with improvement in quality of life (11). Nevertheless, there is a high mortality and morbidity rate associated with hemispherectomy (5,6,12). Complications include subdural hematomas and hydrocephalus, often requiring surgical intervention and ventriculoperitoneal shunting. The age of the patient at the time of surgical intervention is an important factor in development of secondary hydrocephalus, with patients younger than 9 months being more at risk (12). The intracranial space left by resection of a large portion of the brain may be intraoperatively lled with Ringer lactate or may eventually become lled with cerebrospinal uid, but it remains vulnerable to infection and hemorrhage. In our case, the patient developed a large left-sided subdural hematoma a few days after right hemispherectomy. Surgical attempts at decompression failed to prevent progression of a left cerebral infarct due to the extensive intracranial bleeding, and the patient died.
3. 4. 5. 6.
7.
8.
9.
10.
References
1. Flores-Sarnat L. Hemimegalencephaly. I. Genetic, clinical, and imaging aspects. J Child Neurol 2002; 17:373384. 2. Barkovich AJ, Chuang SH. Unilateral megalencephaly: correlation of MR imaging and pathologic 11.
12.
characteristics. AJNR Am J Neuroradiol 1990; 11:523531. Sims J. On hypertrophy and atrophy of the brain. Med Quir Trans 1835; 19:315380. Wolpert SM, Cohen A, Libenson MH. Hemimegalencephaly: a longitudinal MR study. AJNR Am J Neuroradiol 1994; 15:1479 1482. Vigevano F, Bertini E, Boldrini R, et al. Hemimegalencephaly and intractable epilepsy: benets of hemispherectomy. Epilepsia 1989; 30:833 843. Mathis JM, Barr JD, Albright AL, Horton JA. Hemimegalencephaly and intractable epilepsy treated with embolic hemispherectomy. AJNR Am J Neuroradiol 1995; 16:1076 1079. Yagishita A, Arai N, Tamagawa K, Oda M. Hemimegalencephaly: signal changes suggesting abnormal myelination in MRI. Neuroradiology 1998; 40:734 738. Rintahaka PJ, Chugani HT, Messa C, Phelps ME. Hemimegalencephaly: evaluation with positron emission tomography. Pediatr Neurol 1993; 9:21 28. Adamsbaum C, Robain O, Cohen P, Delalande O, Fohlen M, Kalifa G. Focal cortical dysplasia and hemimegalencephaly: histological and neuroimaging correlations. Pediatr Radiol 1998; 28:583590. Woo C, Chuang S, Becker L, et al. Radiologicpathologic correlation in focal cortical dysplasia and hemimegalencephaly in 18 children. Pediatr Neurol 2001; 25:295303. Carreno M, Wyllie E, Bingaman W, Kotagal P, Comair Y, Ruggieri P. Seizure outcome after functional hemispherectomy for malformations of cortical development. Neurology 2001; 57:331333. Di Rocco C, Iannelli A. Hemimegalencephaly and intractable epilepsy: complications of hemispherectomy and their correlation with the surgical techniquea report on 15 cases. Pediatr Neurosurg 2000; 33:198 207.
You might also like
- Pediatric Case of The DayDocument5 pagesPediatric Case of The Dayapi-148100258No ratings yet
- HmesonogrampatternpaperDocument1 pageHmesonogrampatternpaperapi-148100258No ratings yet
- Contralateral Hemimicrencephaly and Clinical-Pathological Correlations in Children With HemimegalencephalyDocument14 pagesContralateral Hemimicrencephaly and Clinical-Pathological Correlations in Children With Hemimegalencephalyapi-148100258No ratings yet
- Clinical Outcomes of Hemispherectomy For Epilepsy in Childhood and AdolescenceDocument11 pagesClinical Outcomes of Hemispherectomy For Epilepsy in Childhood and Adolescenceapi-148100258No ratings yet
- Hemispherectomy - A Schematic Review of The Current TechniquesDocument6 pagesHemispherectomy - A Schematic Review of The Current Techniquesapi-148100258No ratings yet
- HmeandintractableepilepsyDocument4 pagesHmeandintractableepilepsyapi-148100258No ratings yet
- Functional Consequences of HemispherectomyDocument9 pagesFunctional Consequences of Hemispherectomyapi-148100258No ratings yet
- Hme Mrimaging 5childrenDocument5 pagesHme Mrimaging 5childrenapi-148100258No ratings yet
- Functional Hemispherectomy For The Treatment of Intractable SeizuresDocument6 pagesFunctional Hemispherectomy For The Treatment of Intractable Seizuresapi-148100258No ratings yet
- Hemimegalencephaly: A Study of Abnormalities Occurring Outside The Involved HemisphereDocument5 pagesHemimegalencephaly: A Study of Abnormalities Occurring Outside The Involved Hemisphereapi-148100258No ratings yet
- Hme and Epilepsy An OverviewDocument7 pagesHme and Epilepsy An Overviewapi-148100258No ratings yet
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5783)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (890)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (72)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Sheikh Zayed Grand Mosque - Largest in UAEDocument2 pagesSheikh Zayed Grand Mosque - Largest in UAEKyla SordillaNo ratings yet
- Small-Scale Fisheries Co-op ConstitutionDocument37 pagesSmall-Scale Fisheries Co-op ConstitutionCalyn MusondaNo ratings yet
- KD.7.1-WPS OfficeDocument9 pagesKD.7.1-WPS OfficePratista TyasNo ratings yet
- ENGLISH COACHING CORNER MATHEMATICS PRE-BOARD EXAMINATIONDocument2 pagesENGLISH COACHING CORNER MATHEMATICS PRE-BOARD EXAMINATIONVaseem QureshiNo ratings yet
- Final VeganDocument11 pagesFinal Veganapi-314696134No ratings yet
- The NF and BNF Charts from the Trading RoomDocument23 pagesThe NF and BNF Charts from the Trading RoomSinghRaviNo ratings yet
- Horses To Follow: Ten To Follow From Timeform'S Team of ExpertsDocument12 pagesHorses To Follow: Ten To Follow From Timeform'S Team of ExpertsNita naNo ratings yet
- Patanjali PDFDocument11 pagesPatanjali PDFSiddharth GuptaNo ratings yet
- Sales TAX FORMATDocument6 pagesSales TAX FORMATMuhammad HamzaNo ratings yet
- Lecture Notes On Revaluation and Impairment PDFDocument6 pagesLecture Notes On Revaluation and Impairment PDFjudel ArielNo ratings yet
- Cambridge O Level: Agriculture 5038/12 October/November 2020Document30 pagesCambridge O Level: Agriculture 5038/12 October/November 2020Sraboni ChowdhuryNo ratings yet
- Acc121 Exam1 ProblemsDocument4 pagesAcc121 Exam1 ProblemsTia1977No ratings yet
- Hiata 2Document21 pagesHiata 2AnnJenn AsideraNo ratings yet
- Service ManualDocument14 pagesService ManualOlegNo ratings yet
- Komatsu PC01-1 (JPN) 14001-Up Shop ManualDocument217 pagesKomatsu PC01-1 (JPN) 14001-Up Shop Manualhaimay118100% (2)
- EM24DINDSDocument14 pagesEM24DINDSJavaprima Dinamika AbadiNo ratings yet
- Callon & Law (1997) - After The Individual in Society. Lessons On Colectivity From Science, Technology and SocietyDocument19 pagesCallon & Law (1997) - After The Individual in Society. Lessons On Colectivity From Science, Technology and Societysashadam815812No ratings yet
- SheeshDocument31 pagesSheeshfrancisco bonaNo ratings yet
- Preparing For 2024: Election Technology and The Battle Against DisinformationDocument13 pagesPreparing For 2024: Election Technology and The Battle Against DisinformationVerified VotingNo ratings yet
- Environment Health: European Research OnDocument73 pagesEnvironment Health: European Research OnDaiuk.DakNo ratings yet
- Monster c4 Thread Text - Edited (Way Long) Version2 - Ford Muscle Cars Tech ForumDocument19 pagesMonster c4 Thread Text - Edited (Way Long) Version2 - Ford Muscle Cars Tech Forumjohn larsonNo ratings yet
- User'S Manual: 1 - Installation 2 - Technical SpecificationsDocument8 pagesUser'S Manual: 1 - Installation 2 - Technical SpecificationsGopal HegdeNo ratings yet
- Syed Shujauddin 124661163Document3 pagesSyed Shujauddin 124661163shujauddin11No ratings yet
- Haley Sue Exsted FacebookDocument58 pagesHaley Sue Exsted Facebookapi-323808402No ratings yet
- Module 3 - Subsequent To AcquisitionDocument8 pagesModule 3 - Subsequent To AcquisitionRENZ ALFRED ASTRERONo ratings yet
- Adverse Drug Reactions in A ComplementaryDocument8 pagesAdverse Drug Reactions in A Complementaryrr48843No ratings yet
- Easa Ad 2023-0133 1Document6 pagesEasa Ad 2023-0133 1Pedro LucasNo ratings yet
- Corporations Defined and FormedDocument16 pagesCorporations Defined and FormedSheryn Mae AlinNo ratings yet
- User Manual With FAQs - Sales Invoice For Petrol PumpsDocument10 pagesUser Manual With FAQs - Sales Invoice For Petrol PumpsRavindra MittalNo ratings yet
- Fa2prob3 1Document3 pagesFa2prob3 1jayNo ratings yet