Professional Documents
Culture Documents
Chemistry 1411
Uploaded by
AliciaTorresCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Chemistry 1411
Uploaded by
AliciaTorresCopyright:
Available Formats
1
Fundamentals
Part A
Matter and energy
Chemical Principles:
The Quest for Insight
Sixth Edition
Peter Atkins and Loretta Jones
2
Story-Board Fundamentals Part A: Matter and energy
A number is
either exact or
measured
Measuring tools
always make one
guess
Notation is
either scientific
or ordinary
Significant
digits
Rounding
numbers
Adding
rule
Multiplication
rule
International
System of
measurements
Dimensional
analysis
Our first
formula is
density
3
Wiki for Fundamentals Part A: Matter and energy
Observations, hypothesis
Law verses a theory
SI units, symbols and there value
Exact and Counting numbers
Recording numbers with measuring tools
Notation is either ordinary or scientific
Which digits are significant
Rules
Round
Adding
Multiplying
Dimensional analysis
Density
Chemistry is extraordinary
Chemistry is the science of
matter and the changes it can
undergo.
It embraces everything material around us-
the stones we stand on, our food and
silicon to make computer chips and more!
4
Dating 1250 to 850 BC
Early examples of chemistry at work.
5
Copper (Cu) is easily
extracted from its ores.
The Bronze Age followed
the discovery that adding
tin (Sn) to copper made
the metal harder and
stronger.
At one level, chemistry is about matter
and its transformations.
We can actually see the changes, a leaf
changes color in the fall, or magnesium
burns brightly in air.
6
Chemistry at Three Levels
This level is the (1)
macroscopic level, the level
dealing with the properties of
large, visible objects .
At a deeper (2) microscopic level,
chemistry deals with the
rearrangements of atoms.
The third level is the (3) symbolic
level, using terms, chemical symbols
and mathematical equations.
A chemist thinks at the microscopic
level, conducts experiments at the
macroscopic level, and represents
both symbolically.
7
Chemistry at Three Levels
8
Scientists pursue ideas in an ill-defined but effective
way called the scientific method.
There are no strict rules or procedures, and answers
are not always available.
Many properties of matter are not described by
grand laws; superconductivity, a major puzzle,
might be resolved in the future by finding the
appropriate law.
How Science Is Done
9
How Science Is Done
Scientists are either meticulous, or highly creative. The
best scientists are a bit of both.
Scientific methods vary, yet our goal is to collect data
from our observations and measurements .
Patterns in our data, can be stated as a scientific law, a
succinct summary of a wide range of observations.
law
theory
What happens
Why it happens
insight
10
How science progresses
insight data law or theory
Collect date (experiments and measurements)
1807
Daltons atomic
Hypothesis-that
matter consists
of atoms
Hypothesis
interpretation
explaining results
of many
experiments
Theory
Hypo-
thesis
Math
Axioms: propositions
(ground rules) that are not
proved but considered self-
evident are the foundation
for advanced math.
Physical science
calculus
addition
Axioms
Our universe unfolds as
we collect data. We build
laws and theory to
explain our observations
Science relies on math. What is the difference?
11
Atomic Theory & Models
12
,,Atoms are building blocks of matter
Negative electrons are embedded in a
sea of positive charge
Positive charge is located within a central
nucleus
Electrons are in circular orbits with quantized
energy levels
Electrons occupy regions of space whose
shape is described by complex
mathematical equations
Quantum
mechanics
model
13
Branches of Chemistry
Organic: carbon compounds
Inorganic: everything but carbon compounds
Physical: theory and principles
Newer
Biochemistry
Analytical: techniques, devices
Computational
Medical
Chemical Engineering: production
Matter is any object with mass and volume, such as a
planet or the substances that makes up the planet or
everything on the planet;
Only a little over 100 different type of atoms are on our
planet.
Objects not included are shadows, affection, justice.
14
Matter and Energy
In Chemistry Substance is a single, pure form of matter.
15
Matter and Energy
Are they related?
All objects move, except at the hypothetical temperature
zero Kelvin.
A common energy formula:
2
2
shows the
relationship between energy, mass and speed.
We see in many energy equations this mass and motion
relationship.
Matter has mass and volume.
3-states of matter
a) Solids
b) Liquids
c) Gases
Whats the difference between these
three states?
Speed
Atoms begin to oscillate
faster as temperature is
increased.
16
Matter and Energy
17
Properties and Changes
Properties identify
Physical: identifies that material
1. Mass
2. Temp
3. Electrical
4. Volume
Chemical: identifies that chemical process (ability to
change into another chemical)
1. Reduction oxidation potential
2. Solubility
3. Thermodynamics, is it spontaneous
Any characteristic that describes or identify matter is called property
18
Properties and Changes
Changes are an alteration or process
Physical
Solid to a liquid
Liquid to a gas
Solid to a gas
Chemical (transformation process)
Magnesium metal + oxygen gas into the magnesium oxide
Carbohydrates turn into energy + CO
2
+ H
2
O
Extensive properties are
physical properties that
depend on the quantity of
matter (n atoms): Volume &
Mass
19
Intensive and Extensive Properties
Cutting coins in half will give
you half the number of atoms
Same temperature
different volumes.
20
Intensive and Extensive Properties
Intensive properties are
independent of the quantity
of matter: Density &
Temperature
Experimentation and Measurement:
Chemistry is an experimental science
To measure same
quantities such as mass
length, time and
temperature
English System- USA
Metric System -95% of
industrialized word
SI System in 1960 was
based on metric system
Physical
Quantity
SI name of
unit
Meter
system
name
English system
name
mass kilogram
kg
gram
g
pound, ounce
length meter
m
meter
m
mile, feet, inch,
yard..
time second
s
second
s
second
s
Tempera-
ture
Kelvin
K
t
o
Celsius
Fahrenheit
F
21
Chapter 1/22 2012 Pearson Education, Inc.
Experimentation and Measurement: Chemistry
is an experimental science
Systme Internationale dUnits
All other units are derived from these fundamental units.
Very large and very small numbers are indicated
by scientific notation, using exponential format
23
SI System, youll find them in the back binder
Mega
kilo
deci
centi
milli
micro
nano
pico
For Example
M = 10
6
k = 10
3
d = 10
-1
c = 10
-2
m = 10
-3
= 10
-6
n = 10
-9
p = 10
-12
Common SI prefixes
1 Megawatt (wind turbine)
kilometer (distance)
milliliter, syringes
micrometer, cell diameter
nanometer, Chemical bond
picometer, atom diameter
(m = .001)L
mL = .001L
or
1000 mL = L
Conversion unit
milli- is the SI name for the symbol m, and a prefix, the
mathematical representation of milli- is 10
-3
. This is
typically written as m = 10
-3
.
We can combine the SI symbol and number
We can treat this like an algebraic expression
Multiplying the L through the parenthesis
24
Using the SI system for conversions
Write a conversion unit for pico gram
Write a conversion unit for mega watt
Write a conversion unit for seconds in a minute
(p = 10
-12
)
(M = 10
6
)
60 sec = 1 min
g
w
pg = 10
-12
g
Mw = 10
6
w
25
Measurements: Conversions
Conversion Unit: 1000 mL = L
Conversion factors are conversion units written as a
numerator and denominator.
1000
= 1
1 =
1000
Transforming a Conversion Units into a Conversion factor.
26
60 sec = 1 min
pg = 10
-12
g
Mw = 10
6
w
10
12
g
or
10
12
g
Mw
10
6
w
or
10
6
w
Mw
60 sec
1
or
1
60 sec
Rewrite the following as conversion factors, two different
ways.
27
10
-12
gpg
-1
or
28
What are Important Digits
Science is collecting data and reporting our results.
We have to justify our data by reporting the correct
number of digits from our measurements.
We only write the correct number of digits in our reports,
these are called significant figures.
The number of significant figures in our calculations
cannot exceed our data measurements.
You can't generate data from a calculator!
Measurements: Measured
Measured number come from measuring tools.
The problem is measuring tools are not exact ; accuracy is
determined by the manufacture.
1
100
1
1000
30
Recording Measurements
When using a measuring tool
1. Write all the digits you see
2. Make one guess
3. Add units
4.8
4.82
4.82 cm
2 decimal numbers
31
Recording Measurements
When using a measuring tool
1. Write all the digits you see
2. Make one guess
3. Add units
25
25.7
25.7
1 decimal number
32
Recording Measurements
When using a measuring tool
1. Write all the digits you see
2. Make one guess
3. Add units
8.0
8.00
8.00 cm
This number has 3 digits
2 decimal numbers
33
Reading liquid: get eye level with the Bottom of the meniscus
Adhesion
Cohesion
34
Reading liquid: get eye level with the Bottom of the meniscus
What would the volume reading be?
What do you see:
What is your guess:
Final:
16
16.4
16.4 mL
50
40
30
20
10
0
35
Numbers in our data are written in either
1. Ordinary notation
2. Scientific notation
A big advantage of scientific notation is its easier to
write both big and small numbers.
Notation
Ordinary notation
36,000,000
Scientific notation
3.6 10
7
36
How to write scientific notation for a big number.
An example using 36,000,000
Locate the first digit
Place the decimal after the first digit
Write all digits after the first digit
Use the notation 10
number of decimal hops
Notation
36,000,000 3. 3.6 3.6 10
7
7 6 5 4 3 2 1
.
37
How to write scientific notation for a small number.
An example using 0.000089
Locate the first digit
Place the decimal after the first digit
Write all digits after the first digit
Use the notation 10
number of decimal hops
Notation
0.000089 8. 8.9 8.9 10
-5
Here we use a -
to indicate a
number smaller
than 1
1 2 3 4 5
38
Write the following ordinary numbers in scientific
notation.
Notation
1.22 10
2
7.801 10
4
9.59 10
8
9.5 10
-4
1.254 10
-1
1 10
0
122
78,010
959,000,000
0.00095
0.1254
1
39
digits
There are two types of digits
1. Exact
2. Measured
Exact are either:
Counting (Im holding 5 fingers) or are,
Definitions (60 seconds = 1 minute), these have an infinite
number of digits, so 5 fingers means 5.00000 with an
number of zeros.
All other numbers are measured with a measuring tool,
hence are inaccurate.
40
Measurements: Exact
Exact Numbers
1. Counting
How many raised fingers are on the hand?
How many piglets are there?
2. Definitions
60 minutes in 1 hour
100 pennies in 1 dollar
1000 milliliters in 1 liter
5 fingers on one hand
Exact numbers have infinite () precision!
60.0000000 minutes = 1 dollar ( number of zeros)
41
Significant Figure (SF)
appendix 1C
Rules for reporting results from a calculation
1. Digits
2. Rounding
3. Addition & Subtraction
4. Multiplication & Division
We will focus on the difficult concept Zero.
9547 cm (4 SF)
4.29 g (3 SF)
12 in = 1 ft ( number of SF)
Different kinds of Zeros
Captive: 807 miles
Trailing: 600.
600
Leading: 0.00010
(3 SF) all the zeros count
(3 SF) decimals make zeros SF
(1 SF) without decimals zeros are not SF
(2 SF) leading zeros are not SF
42
Significant Figure (SF)
Remember: zeros at the end with a decimal count
How many SF in the following
725
6001
9010
9010.
0.00680
1 ft = 12 in
3 SF
4 SF
3 SF no decimal
4 SF decimal
3 SF leading no, trailing yes
, its a definition
43
Significant Figure (SF)
44
Significant Figure (SF)
How many SF in the following
50.0000
0000000.01
00023.0200
0.0020200
4500000000
45000.0000
1000 mL = 1 L
6 SF
1 SF leading no
6 SF leading no, sandwich and trailing yes
5 SF
2 SF
9 SF
, its a definition
45
Significant Figure: Rounding
Rounding off in calculations, round up if the last digit is
above 5 and round down if it is below 5.
For numbers ending in 5, always round to the nearest
even number. 0 is considered as even number.
2.35 rounds to 2.4
2.65 rounds to 2.6
In a calculation with multiple steps, round off only in
the final step.
Round the following into a 3 SF number
5.640
900810
8887
0.000021
20
9
9000
89019.0
5.64
901000
8890
0.0000210
20.0
9.00
9.00 x 10
3
8.90 x 10
4
46
Round the following into a 4 SF number
335.64
80236
18,000
0.002
7
44102.02
1999.6
335.6
80240
1.800 x 10
4
.002000
7.000
4.410 x 10
4
2000.
47
255 32.7 320000
+34.0 - 0.0049 + 12.5
289.0 32.6951 320012.5
289 32.7 320000 (1 and 2)
48
Significant Figure: Addition & Subtraction
When adding or subtracting, make sure that the number
of decimal places in the result is the same as the smallest
number of decimal places in the data.
Addition or subtraction:
1. The answer cant have more digits to the
right of the decimal point than any of the original numbers.
2. The number of significant figures in the calculations cannot exceed
the data measurements.
1200 500. 100
+250 + 0.00021 - 98.5
1450 500.00021 001.5
Recorded Answers
1500 500. 2
49
Significant Figure: Addition & Subtraction
More practice:
Stop at the least SF digit
The zero does not count
since there is no decimal.
Must be whole number
6600 10.0 10 10
+25.0 - 9.85 - 9.85 -4.85
6625.0 .15 00.15 5.15
Recorded Answers
6600 .2
0
50
Significant Figure: Addition & Subtraction
More practice:
5
5.02 89,665 0.10 = 45.0118 = 45
5.892 6.10 = 0.96590 = 0.966
51
Significant Figure: Multiplication & Division
When multiplying or dividing, make sure that the number
of significant figures in the result is the same as the
fewest number of significant figures in the data (the
least accurate).
1.01 0.12 53.51 96 = 0.067556 = 0.068
56.55 0.920 34.2585 = 1.51863 = 1.52
52
Significant Figure: Multiplication & Division
53
Dimensional Analysis
The next section introduces a method for solving
problems called Dimension analysis.
The method uses conversion factors.
Conversion factors must be written with a numerator
and denominator.
10
6
10
12
60
54
1
=
1
1
2
2
1
=
When using a conversion factor, treat the units just
like algebraic quantity: they are multiplied or canceled in
the normal way.
The most important reason for doing this is to see how
units cancel.
Dimensional Analysis
55
Dimensional Analysis
Comment:
Students can benefit by using dimensional
analysis as a way of recognizing how to organize
data.
Following a few simple guidelines, repeatedly, will
make problem-solving less of a chore and more of
an art-form.
Dimensional Analysis
What are the units in the answer:
Write out given and missing information:
Starting in the numerator, writing the unit of the answer:
Cancel each unwanted unit not appearing in the answer.
wanted unit
a
= wanted unit
Procedure for a typical dimensional analysis problem.
wanted unit
wanted unit/a, a/b, b/c, c/1
wanted unit
a
Question: Find wanted unit.
Starting with our wanted unit, our answer will have the correct unit
a
b
b
c
c
1
= L
Final answer
57
Example A.I Suppose we want to convert a volume of 1.7 qt into liters.
What are the units in the answer:
Write out given and missing information:
Starting in the numerator, writing the unit of the answer:
Cancel each unwanted unit not appearing in the answer.
1.7 qt
1
=
Liters, L
1.7 qt, 1 qt = 0.946 L
0.946 L
1 qt
1.60820 L
Our measured number with the least SF is 1.7 or 2SF, so
1.6 L
0.946 L
1 qt
= cm
58
Self-Test A1.A Express the height of a person 6.0 feet tall in
centimeters.
Final
answer
What are the units in the answer:
Write out given and missing information:
Starting in the numerator, writing the unit of the answer:
Cancel each unwanted unit not appearing in the answer.
cm
6.0 ft, 12 in = 1 ft,
2.54 cm = 1 in
Our measured number with the least SF is 6.0 or 2SF, so
180 cm 182.880 cm
250. g
ounces or oz
1 kg = 2.2 lbs
1000 g = 1 kg
16 oz = 1 lb
= oz
16 oz
1 lb
2.2 lb
1kg
1kg
1000g
Our measured number with the least SF is 250. or 3SF
59
Self-Test A.1B Express the mass in ounces of a 250. g package of
breakfast cereal.
What are the units in the answer:
Write out given and missing information:
Starting in the numerator, writing the unit of the answer:
Cancel each unwanted unit not appearing in the answer.
8.80000 oz
Final
answer
8.80 oz
250.g
1
=
Self-test A.2A Express a density of 6.5 gmm
-3
in micrograms per
nanometer cubed (gnm
-3
).
What are the units in the answer:
Write out given and missing information:
6.5 gmm
-3
gnm
-3
1 g = 10
-6
g
1 mm = 10
-3
m
1 nm = 10
-9
m
=
g
nm
3
1 g
10
6
g
6.5 g
mm
3
1 mm
10
3
m
10
9
m
1 nm
=
Our measured number with the least SF is 6.5 or 2SF
Match the units, then cube.
10
9
m
1 nm
3
=
1 mm
10
3
m
3
6.5 10
12
g
nm
3
The entire calculation is always preformed in your calculator as one
continuous string of operations; it is incorrect, and you will have a
greater chance of producing errors by writing separate numerators
and denominators proceeded by a calculation.
61
Self-test A.2B Express an acceleration of 9.81 ms
-2
in kilometers per
hour squared (kmhr
-2
).
What are the units in the answer:
Write out given and missing information: 9.81 ms
-2
kmhr
-2
1 km = 1000 m
1 hr = 3600 s
=
km
hr
2
1 km
1000 m
9.81 m
s
2
3600 s
1 hr
Our measured number with the least SF is 9.81 or 3SF
Match the units, then cube.
3600 s
1 hr
2
=
1.27 10
5
km
hr
2
Precise & Accurate Precise
Accurate
62
Neither precise
nor accurate
Chapter 1/63 2012 Pearson Education, Inc.
Mass and Its Measurement
Mass: Amount of matter in an
object
Weight: Measures the force with
which gravity pulls on an object
Weighting means that we are
measuring mass
SI Unit: kg, g, mg, microgram (g)
Standard Unit: cylindrical bar of
Platinum-iridium alloy
Chapter 1/64 2012 Pearson Education, Inc.
Length and Its Measurement
Meter (standard unit)
1 m =100 cm =1000 mm =1 x10
6
m = 1 x 10
9
nm =1 x10
-3
km
1790: One ten-millionth of the distance from the equator to the North pole along a
meridian running through Paris, France
1889: Distance between two thin lines on a bar of platinum-iridium alloy stored near
Paris, France
1983: The distance light travels in a vacuum in 1/299,792,458 of a second (c = 3.0 x
10
8
m/s)
2012 Pearson Education, Inc. 65
Insert Figure 1.5 p13
(As big as possible, prefer to start at top)
Degree(unit) Size
and zero-point
Chapter 1/66 2012 Pearson Education, Inc.
Temperature and Its Measurement
(
o
F - 32
o
F)
5
o
C
9
o
F
o
C =
o
C + 32
o
F
9
o
F
5
o
C
o
F =
K =
o
C + 273.15
Degree size and
zero-point
adjustment
zero-point
adjustment
5
o
C = 5
o
C X 1.8
o
F/
o
C + 32
o
F = 41
o
F
5
o
C = 5
o
C + 273.15 = 278 K
Chapter 1/67 2012 Pearson Education, Inc.
Derived Units: Volume and Its
Measurement
2012 Pearson Education, Inc. 68
Volume, the amount of space occupied by an object, is
measured in SI units by the cubic meter (m
3
)
1 m
3
= 1000 L
69
Density: intensive independent of the number of atoms
Density: is direct relationship between
mass and volume (both extensive)
=
3
,
Interestingly, multiplying or dividing two extensive
(dependent on the number of atoms) properties must give
you an intensive (independent of the number of atoms)
property.
Derived Units: Density and Its
Measurement
Chapter 1/70 2012 Pearson Education, Inc.
Density is the amount of matter in a certain amount of space
solids- cm
3
d --- g/ cm
3
liquids- mL d -----g/mL
gases- L
Typical volume units
Determining Density for Regular
Objects
V
cube
= a x a x a = a
3
V = a x b x c
parallelepiped
V
cylinder
= r
2
h
V
sphere
= 4 r
3
/3 = 4/3 x r
3
V s
phere
= 1/6 x d
3
Determining Density for Irregular
Objects
Chapter 1/72 2012 Pearson Education, Inc.
Chapter 1/73 2012 Pearson Education, Inc.
40.0 mL
1 figure after decimal
Compare our answer to either gold or fools gold.
112 g, 35.8 mL, 41.6 mL
g
mL
=
g
mL
112 g
1
1
41.6 35.8 mL
74
You discovered a piece of matter that looks like gold, d = 19.28 g/cm
3
,
but it may be fool's gold, d = 5.02 g/cm
3
. The sample mass is 112
grams. When placed in a graduated cylinder containing 35.8 mL of
water, the water level rises to 41.6 mL. Calculate the density of the
sample and identify it.
What are the units in the answer:
Write out given and missing information:
= 19
g
mL
which is gold
1
5.8 mL
41.6 - 35.8 = 5.8 a 2SF so our final answer is 19
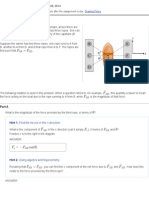
F=ma
=
if m then F
75
Force
Energy is work
1 heart beat = 1 J
There are three contributions to energy: kinetic energy,
potential energy and electromagnetic energy.
1 = 1
2
2
76
Energy
Kinetic is the energy that a body possesses due to
motion.
Potential is the energy that a body possesses due to its
position in a field of force.
Electromagnetic is the energy due to attractions and
repulsions between electric charges.
=
1
2
2
4
0
m = mass
= velocity
g = gravitational force
h = height
q = charge
77
Energy
=
1
2
2
To raise your book
from floor to
tabletop requires
approximately 14 J.
of energy. The same
energy released if
the book fell to the
floor.
Fast moving atoms
exert a force, by
pushing against the
atmosphere.
78
Energy
Kinetic is the energy of a moving body
Energy
=
The potential energy of a mass
in a gravitational field is
proportional to its height h. The
starting position on the floor
correspond to zero potential
energy.
79
Potential is static, energy that a non-moving body
possesses due to its position in a field of force.
Energy
=
Someone of mass 65 kg walks up a
flight of stairs between two floors of
a building that are separated by 3.0
m. What is the change in potential
energy of the person?
=65kg 9.81 ms
-2
3.0m = 1.9 kJ
80
Potential is static, energy that a non-moving body
possesses due to its position in a field of force.
2
4
0
The energy of electric charges
found atomic nuclei and
electrons are charged.
Coulomb potential energy of
two opposite charges (one
red the other green)
At the bottom, potential
energy decreases.
81
Energy
Electromagnetic is the attractive and
repulsive energy between electric charges
Electromagnetic fields (photons)
carry through space as radio
waves, light waves and x-rays.
Moving charged particles
generate an oscillating electric
field and an oscillating magnetic
field.
82
Electromagnetic
The crucial distinction is that electric fields affect charged
particles whether they are moving or still, and magnetic
fields affect only moving charged particles.
Total Energy = KE + PE
A ball thrown up has high KE and
zero PE. At the top of its flight, it has
zero KE and high PE. On the return,
its KE rises and its PE approaches
zero again.
On impact its energy is dissipated as
thermal motion, the chaotic,
random motion of atoms and
molecules.
83
E
T
= E
K
+ E
P
Totaling KE and PE of Earth and the ball are the same; Law of
conservation of energy, is energy can be neither created nor
destroyed.
You might also like
- Experimental Methods and Instrumentation for Chemical EngineersFrom EverandExperimental Methods and Instrumentation for Chemical EngineersNo ratings yet
- MODULES-IN-PHYSICAL-SCIENCE WithnyouuuDocument10 pagesMODULES-IN-PHYSICAL-SCIENCE WithnyouuuHesoyam HesoyamNo ratings yet
- Module 2: The Scientific Method and Matter: CHEM 208Document56 pagesModule 2: The Scientific Method and Matter: CHEM 208Nikita DaryananiNo ratings yet
- Physics For Scientists and EngineersDocument46 pagesPhysics For Scientists and EngineersloloAG100% (1)
- Physics For Scientists and EngineersDocument45 pagesPhysics For Scientists and EngineersSara Quintana HornaNo ratings yet
- What Is Physics?: Unit 1. Measuring 3º ESO Physics and ChemistryDocument14 pagesWhat Is Physics?: Unit 1. Measuring 3º ESO Physics and ChemistryJose Luis Jorge MartínNo ratings yet
- PHYSICS 1 Lecture For Midterm PDFDocument27 pagesPHYSICS 1 Lecture For Midterm PDFTrisha Mae Fabales CanlasNo ratings yet
- Units of Conversion, Significant Figures, Scientific Notation and TemperatureDocument34 pagesUnits of Conversion, Significant Figures, Scientific Notation and TemperatureBeatrice AgustinNo ratings yet
- General Physics 1 NotesDocument10 pagesGeneral Physics 1 NotesMiguel Luis JumawanNo ratings yet
- Introduction to ChemistryDocument13 pagesIntroduction to ChemistryMichael Jerwin AbellaNo ratings yet
- Measurement & Significant FiguresDocument65 pagesMeasurement & Significant FiguresJim Marco100% (1)
- Atoms and MoleculesDocument12 pagesAtoms and MoleculesKshithij R KikkeriNo ratings yet
- Unit 1 Physical Quantities and MeasurementDocument30 pagesUnit 1 Physical Quantities and MeasurementZaraNo ratings yet
- Spectrum 01Document39 pagesSpectrum 01Chester Allan Miguel-EduriaNo ratings yet
- FAP0015 Ch01 MeasurementDocument35 pagesFAP0015 Ch01 MeasurementFadhil Muhammad BarzaniNo ratings yet
- Book II Chapter 02Document19 pagesBook II Chapter 02Anh MongNo ratings yet
- General Physics 1: Quarter 1 - Week 1Document37 pagesGeneral Physics 1: Quarter 1 - Week 1lerkhe toribioNo ratings yet
- PHY 111 IntroductionDocument49 pagesPHY 111 IntroductionijaiyajuwonNo ratings yet
- 01 IntroductionDocument10 pages01 IntroductionAlexys BazanNo ratings yet
- Chapter 1: Introduction: Physics (In Latin AlphabetDocument6 pagesChapter 1: Introduction: Physics (In Latin AlphabetSevim KöseNo ratings yet
- 1.1 1.2 IntroductionDocument24 pages1.1 1.2 IntroductionbellarosyNo ratings yet
- Spectrum 01Document39 pagesSpectrum 01Cedie LooNo ratings yet
- Basic Concept of Chemistry 1Document15 pagesBasic Concept of Chemistry 1api-233404189No ratings yet
- Introduction To Physics and MeasurementDocument9 pagesIntroduction To Physics and MeasurementNishant CortezNo ratings yet
- PHYSICS MEASUREMENT FUNDAMENTALSDocument20 pagesPHYSICS MEASUREMENT FUNDAMENTALSMarga BautistaNo ratings yet
- Physics - 1 Iit MaterialDocument1,438 pagesPhysics - 1 Iit MaterialSHK sirasapalliNo ratings yet
- CHEM 111 NoteDocument35 pagesCHEM 111 NoteLunaNo ratings yet
- Chapter 2: Standards For Measurement: 2.1 Significant FiguresDocument5 pagesChapter 2: Standards For Measurement: 2.1 Significant Figureschin dyNo ratings yet
- 20210413-Physics Class Notes From Slides-1Document9 pages20210413-Physics Class Notes From Slides-1GeneNo ratings yet
- Base Quantities and Derived QuantitiesDocument31 pagesBase Quantities and Derived Quantitiesdirza82100% (1)
- Complete Notes On 9th Physics by Asif RasheedDocument53 pagesComplete Notes On 9th Physics by Asif RasheedSajid HusnainNo ratings yet
- CHM113 CH 1 (Chang) HandoutsDocument10 pagesCHM113 CH 1 (Chang) HandoutsHsiung Wei WeiNo ratings yet
- CHEMISTRY IntroDocument76 pagesCHEMISTRY IntroLeanneNo ratings yet
- CBSE Class 11 Physics Chapter 2 Units and Measurement Revision NotesDocument63 pagesCBSE Class 11 Physics Chapter 2 Units and Measurement Revision Notesrazi sharifNo ratings yet
- Introduction To ChemistryDocument46 pagesIntroduction To Chemistrypaul garcia100% (1)
- Chemistry Chapter 1-4 Online NotesDocument37 pagesChemistry Chapter 1-4 Online NoteskellychensterNo ratings yet
- Fundamental concepts of chemistryDocument30 pagesFundamental concepts of chemistryAravindNo ratings yet
- Chapter 1wDocument26 pagesChapter 1wShelley HernandezNo ratings yet
- Adrian Duncan-Introduction To Chemical Engineering Processes-Global Media (2009)Document177 pagesAdrian Duncan-Introduction To Chemical Engineering Processes-Global Media (2009)Frank A.No ratings yet
- Topic 1.1 - Measurements in PhysicsDocument42 pagesTopic 1.1 - Measurements in PhysicsShrey JainNo ratings yet
- 1.3 Introduction To Chemical Engineering Processes PDFDocument11 pages1.3 Introduction To Chemical Engineering Processes PDFEddie CastilloNo ratings yet
- General Science Concepts and LawsDocument30 pagesGeneral Science Concepts and Lawssenior highNo ratings yet
- ScienceDocument32 pagesScienceREGINE DANIELLE CAAMPUEDNo ratings yet
- Physical Quantities and DimensionsThe proposed title "TITLE Physical Quantities and DimensionsDocument37 pagesPhysical Quantities and DimensionsThe proposed title "TITLE Physical Quantities and DimensionsTutor_KLNo ratings yet
- Lecture 1 - Chapter 1 Part 1Document28 pagesLecture 1 - Chapter 1 Part 1Mircea PanteaNo ratings yet
- MMM MMMMM MM MMMM YY YDocument8 pagesMMM MMMMM MM MMMM YY YEunae ChungNo ratings yet
- Physical Chemistry Guide to Units, Moles and CalculationsDocument40 pagesPhysical Chemistry Guide to Units, Moles and CalculationsHoney Nhassie Marie GonzagaNo ratings yet
- Fisica 1 TemasDocument36 pagesFisica 1 TemasUsuario 1023No ratings yet
- 11 Chemistry Notes Ch01 Some Basic Concepts of ChemistryDocument16 pages11 Chemistry Notes Ch01 Some Basic Concepts of ChemistryAnudeex ShettyNo ratings yet
- Laboratory Manual Physics of Engineers: Engr. Danielle Joy L. AlcantaraDocument38 pagesLaboratory Manual Physics of Engineers: Engr. Danielle Joy L. AlcantaraSusan LandichoNo ratings yet
- Chemistry: A Science for the 21st CenturyDocument6 pagesChemistry: A Science for the 21st Centuryasianpanda14No ratings yet
- Chapter 1Document43 pagesChapter 1ritonga01No ratings yet
- Measurements in ChemistryDocument27 pagesMeasurements in ChemistryAlyssa AmigoNo ratings yet
- Module 1 Chapter 1 in MechanicsDocument8 pagesModule 1 Chapter 1 in MechanicsclarisseNo ratings yet
- Physics For Secondary SchoolsDocument238 pagesPhysics For Secondary Schoolsgeoffrey osuriNo ratings yet
- Scince (Assement-1)Document4 pagesScince (Assement-1)ARAF ABDULLAHNo ratings yet
- Module 1Document18 pagesModule 1let's skip thisNo ratings yet
- The Study of Matter, Energy, and The Interactions Between ThemDocument50 pagesThe Study of Matter, Energy, and The Interactions Between ThemAllyna BautistaNo ratings yet
- Physics For Scientists and EngineersDocument36 pagesPhysics For Scientists and Engineersapi-26417140No ratings yet
- EXERCISESDocument13 pagesEXERCISESAliciaTorresNo ratings yet
- Quick Studies CH 5Document8 pagesQuick Studies CH 5AliciaTorresNo ratings yet
- Chapter 6 Questions AccountingDocument3 pagesChapter 6 Questions AccountingAliciaTorresNo ratings yet
- CH 06 HWDocument40 pagesCH 06 HWAliciaTorres76% (33)
- Boat Statics Forces SolvedDocument67 pagesBoat Statics Forces SolvedAliciaTorres0% (1)
- UntitledDocument253 pagesUntitledKathy LuoNo ratings yet
- 33 11 SubstationDocument21 pages33 11 SubstationRamdevNo ratings yet
- QuizDocument14 pagesQuizmnmusorNo ratings yet
- Go Off-Grid with Solar PV GuideDocument3 pagesGo Off-Grid with Solar PV GuideMirela VihacencuNo ratings yet
- Specific Objectives: Pressure QuestionsDocument6 pagesSpecific Objectives: Pressure QuestionsBadmind JnrNo ratings yet
- Transpiration: Prepared By: Concepcion, Ada - Trinidad, Lester - Tolon, ChristianDocument17 pagesTranspiration: Prepared By: Concepcion, Ada - Trinidad, Lester - Tolon, ChristiannimhaNo ratings yet
- CV Khaled YounesDocument8 pagesCV Khaled YounesKhaled YounesNo ratings yet
- Oxygen Reduction ReactionDocument6 pagesOxygen Reduction ReactionYabisira ayeleNo ratings yet
- Measure Precipitation with a Rain GaugeDocument5 pagesMeasure Precipitation with a Rain GaugeKathy SarmientoNo ratings yet
- Wetland BookDocument295 pagesWetland BookChanel Tri HandokoNo ratings yet
- Biochemical CycleDocument39 pagesBiochemical CycleShubhangi BNo ratings yet
- Vishal Shah-InvestnebtDocument7 pagesVishal Shah-InvestnebtRobinNo ratings yet
- Earth System History 4th Edition Stanley Test BankDocument10 pagesEarth System History 4th Edition Stanley Test Bankpanabase.trevat.puhh6e100% (19)
- Scuttlers, Black Goo, and The Biogenic FieldDocument27 pagesScuttlers, Black Goo, and The Biogenic FieldIan Beardsley100% (1)
- Science 9 - Q2 - Week2 - Melc 5Document10 pagesScience 9 - Q2 - Week2 - Melc 5Rhyan Zero-four BaluyutNo ratings yet
- Physics O LevelDocument32 pagesPhysics O LevelCollins JimNo ratings yet
- Code No: 45005Document4 pagesCode No: 45005SRINIVASA RAO GANTANo ratings yet
- Vh3369 EnglishDocument12 pagesVh3369 EnglishFabio ENo ratings yet
- Artificial EvaporationDocument29 pagesArtificial EvaporationMANISH PARDHINo ratings yet
- 124HarnessingThePower HarnessingThePowerDocument5 pages124HarnessingThePower HarnessingThePower.No ratings yet
- Blue Book PIPEDocument55 pagesBlue Book PIPEJohnMichaelCabungcalNo ratings yet
- ResumeDocument5 pagesResumeAllright ShitNo ratings yet
- Informe 11Document31 pagesInforme 11JHON ALBERT CASTILLO VENTOCILLANo ratings yet
- Geotechnical Formulas and Conversion FactorsDocument35 pagesGeotechnical Formulas and Conversion Factorsdrh31415100% (1)
- Method A7 The Determination of The Maximum Dry Density and Optimum Moisture Content of Gravel, Sand & SoilDocument11 pagesMethod A7 The Determination of The Maximum Dry Density and Optimum Moisture Content of Gravel, Sand & SoilJohn PavlakisNo ratings yet
- A Premium Institute For CBSE, NEET & JEEDocument33 pagesA Premium Institute For CBSE, NEET & JEEZUHAIB KAMALNo ratings yet
- Mineral Grains, Dimples, and Hot Volcanic Organic Streams: Dynamic Geological Backstage of Macromolecular EvolutionDocument12 pagesMineral Grains, Dimples, and Hot Volcanic Organic Streams: Dynamic Geological Backstage of Macromolecular EvolutionВладимир КондратенкоNo ratings yet
- Mass Weight Density NotesDocument5 pagesMass Weight Density NotesMian Mian SuthidaNo ratings yet
- Bahasa Inggris Kelas XI IPA/ XI IPS: Name: Class: DateDocument7 pagesBahasa Inggris Kelas XI IPA/ XI IPS: Name: Class: DateChristevanNo ratings yet
- P. C. Kreijger (Ed.), Adhesion Problems in The Recycling of Concrete © Plenum Press, New York 1981Document2 pagesP. C. Kreijger (Ed.), Adhesion Problems in The Recycling of Concrete © Plenum Press, New York 1981Raji BandaraNo ratings yet
- Coating and Drying Defects: Troubleshooting Operating ProblemsFrom EverandCoating and Drying Defects: Troubleshooting Operating ProblemsRating: 5 out of 5 stars5/5 (1)
- Is That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeFrom EverandIs That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeRating: 4.5 out of 5 stars4.5/5 (3)
- Science Goes Viral: Captivating Accounts of Science in Everyday LifeFrom EverandScience Goes Viral: Captivating Accounts of Science in Everyday LifeRating: 5 out of 5 stars5/5 (1)
- Chemistry for Breakfast: The Amazing Science of Everyday LifeFrom EverandChemistry for Breakfast: The Amazing Science of Everyday LifeRating: 4.5 out of 5 stars4.5/5 (14)
- The Periodic Table: A Very Short IntroductionFrom EverandThe Periodic Table: A Very Short IntroductionRating: 4.5 out of 5 stars4.5/5 (3)
- The Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsFrom EverandThe Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsRating: 4 out of 5 stars4/5 (146)
- The Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableFrom EverandThe Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableRating: 3.5 out of 5 stars3.5/5 (22)
- Essential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilFrom EverandEssential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilRating: 5 out of 5 stars5/5 (1)
- Monkeys, Myths, and Molecules: Separating Fact from Fiction in the Science of Everyday LifeFrom EverandMonkeys, Myths, and Molecules: Separating Fact from Fiction in the Science of Everyday LifeRating: 4 out of 5 stars4/5 (9)
- An Introduction to the Periodic Table of Elements : Chemistry Textbook Grade 8 | Children's Chemistry BooksFrom EverandAn Introduction to the Periodic Table of Elements : Chemistry Textbook Grade 8 | Children's Chemistry BooksRating: 5 out of 5 stars5/5 (1)
- It's Elemental: The Hidden Chemistry in EverythingFrom EverandIt's Elemental: The Hidden Chemistry in EverythingRating: 4 out of 5 stars4/5 (10)
- Organic Chemistry for Schools: Advanced Level and Senior High SchoolFrom EverandOrganic Chemistry for Schools: Advanced Level and Senior High SchoolNo ratings yet
- The Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsFrom EverandThe Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsRating: 5 out of 5 stars5/5 (3)
- Chemistry for Breakfast: The Amazing Science of Everyday LifeFrom EverandChemistry for Breakfast: The Amazing Science of Everyday LifeRating: 4.5 out of 5 stars4.5/5 (90)
- Chemical Elements Pocket Guide: Detailed Summary of the Periodic TableFrom EverandChemical Elements Pocket Guide: Detailed Summary of the Periodic TableNo ratings yet
- Napoleon's Buttons: 17 Molecules That Changed HistoryFrom EverandNapoleon's Buttons: 17 Molecules That Changed HistoryRating: 4 out of 5 stars4/5 (25)
- Guidelines for Asset Integrity ManagementFrom EverandGuidelines for Asset Integrity ManagementRating: 5 out of 5 stars5/5 (1)
- Monkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeFrom EverandMonkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeRating: 4 out of 5 stars4/5 (1)
- The History of Chemistry (Vol.1&2): Complete EditionFrom EverandThe History of Chemistry (Vol.1&2): Complete EditionRating: 1 out of 5 stars1/5 (1)
- Chemistry: a QuickStudy Laminated Reference GuideFrom EverandChemistry: a QuickStudy Laminated Reference GuideRating: 5 out of 5 stars5/5 (1)
- Perfume Engineering: Design, Performance and ClassificationFrom EverandPerfume Engineering: Design, Performance and ClassificationRating: 4 out of 5 stars4/5 (5)